当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination and Analysis of the Solubility of CaSO4·2H2O and α-CaSO4·0.5H2O in Formamide Aqueous Solutions at T = 303.15–363.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-08-29 , DOI: 10.1021/acs.jced.0c00205 Bingjie Yu 1 , Shanshan Miao 1 , Yujia Zhang 1 , Yongsheng Ren 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-08-29 , DOI: 10.1021/acs.jced.0c00205 Bingjie Yu 1 , Shanshan Miao 1 , Yujia Zhang 1 , Yongsheng Ren 1
Affiliation
|
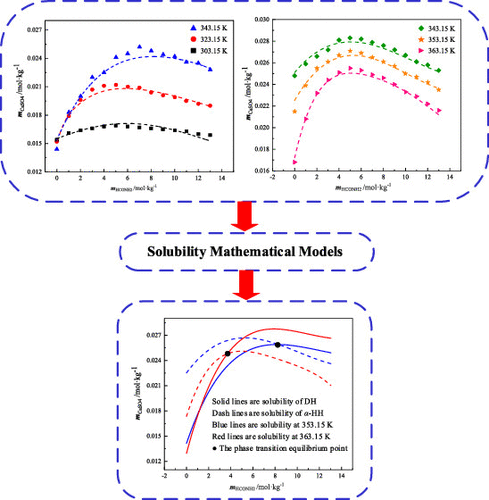
The solubility of calcium sulfate dihydrate (DH) and α-calcium sulfate hemihydrate in formamide aqueous solutions with 0–13 m formamide at temperatures from 303.15 to 363.15 K was measured by the dissolution equilibrium method. Formamide has a solubilization effect similar to the salt effect; the solubility of calcium sulfate dihydrate and α-calcium sulfate hemihydrate first increases significantly as the concentration of formamide aqueous solution increases. When the solubility reaches the maximum value, it decreases gradually. The modified Apelblat–Jouyban–Acree model shows good agreement with experimental values; hence, it may be suitable in describing the solubility of calcium sulfate dihydrate and α-calcium sulfate hemihydrate in the formamide aqueous solution at the temperatures ranging from 303.15 to 363.15 K under 88.35 kPa. On comparing the solubility of calcium sulfate dihydrate and α-calcium sulfate hemihydrate in the formamide aqueous solution, it was found that the solubility of α-calcium sulfate hemihydrate is lower than that of calcium sulfate dihydrate, being above 8.3418 and 3.7286 mol·kg–1 when the temperatures are 353.15 and 363.15 K, respectively, which is beneficial to the conversion of calcium sulfate dihydrate into α-calcium sulfate hemihydrate.
中文翻译:

确定和的CaSO的溶解度分析4 ·2H 2 O和α-的CaSO 4 ·0.5H 2 ○在甲酰胺水溶液于Ť = 303.15-363.15ķ
二水合硫酸钙(DH)和半水合α-硫酸钙在0-13 m的甲酰胺水溶液中的溶解度通过溶解平衡法测量了在303.15至363.15 K温度下的甲酰胺。甲酰胺具有类似于盐的增溶作用。随着甲酰胺水溶液浓度的增加,二水合硫酸钙和α-半水合硫酸钙的溶解度首先显着增加。当溶解度达到最大值时,它逐渐降低。修改后的Apelblat–Jouyban–Acree模型与实验值显示出良好的一致性。因此,它可能适合描述在88.35 kPa下303.15至363.15 K范围内的温度下,二水合硫酸钙和α-半水合硫酸钙在甲酰胺水溶液中的溶解度。在比较二水硫酸钙和α-半水硫酸钙在甲酰胺水溶液中的溶解度时,当温度分别为353.15和363.15 K时为–1,这有利于将二水合硫酸钙转化为α-半水合硫酸钙。
更新日期:2020-09-10
中文翻译:

确定和的CaSO的溶解度分析4 ·2H 2 O和α-的CaSO 4 ·0.5H 2 ○在甲酰胺水溶液于Ť = 303.15-363.15ķ
二水合硫酸钙(DH)和半水合α-硫酸钙在0-13 m的甲酰胺水溶液中的溶解度通过溶解平衡法测量了在303.15至363.15 K温度下的甲酰胺。甲酰胺具有类似于盐的增溶作用。随着甲酰胺水溶液浓度的增加,二水合硫酸钙和α-半水合硫酸钙的溶解度首先显着增加。当溶解度达到最大值时,它逐渐降低。修改后的Apelblat–Jouyban–Acree模型与实验值显示出良好的一致性。因此,它可能适合描述在88.35 kPa下303.15至363.15 K范围内的温度下,二水合硫酸钙和α-半水合硫酸钙在甲酰胺水溶液中的溶解度。在比较二水硫酸钙和α-半水硫酸钙在甲酰胺水溶液中的溶解度时,当温度分别为353.15和363.15 K时为–1,这有利于将二水合硫酸钙转化为α-半水合硫酸钙。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号