Chemosphere ( IF 8.1 ) Pub Date : 2020-08-28 , DOI: 10.1016/j.chemosphere.2020.128142 Xingyun Huang , Ying Peng , Jing Xu , Feng Wu , Gilles Mailhot
|
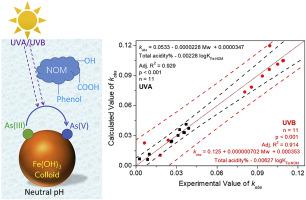
Iron species have essential influence on the environmental/geochemical behaviors of arsenic species in water and soil. Colloidal ferric hydroxide (CFH) induces photooxidation of arsenite (As(III)) to arsenate (As(V)) in water at neutral pH through surface complexation and ligand-to-metal charge transfer (LMCT). However, the effect of the co-existing natural organic matter (NOM) on the complexation-photolysis in this process has remained unclear. In the present work, the photooxidation of As(III) induced by CFH was investigated in the presence of various carboxylic acids and polyphenols as simple model compounds of NOM. Two different light sources of ultraviolet A (UVA) (λmax = 365 nm) and ultraviolet B (UVB) (λmax = 313 nm) were used for photooxidation treatment of the experimental ternary system and the control binary system respectively. The obtained results demonstrated that all investigated NOM inhibited the photooxidation of As(III) in the As(III)/CFH system at pH 7. Moreover, the correlation analysis between the pseudo-first order rate constant kobs and various property parameters of NOM showed that the stable constant for the complexation between Fe(III) and NOM (logKFe-NOM) as well as the molecular weight of NOM and the percentages of total acidity of NOM exhibited significant correlations. A simple quantitative structure-activity relationship (QSAR) model was established between kobs and these three parameters utilizing a multiple linear regression method, which can be employed to estimate the photooxidation efficiency of As(III) in the presence of ferric iron and NOM. Thus, the present work contributes to the understanding of the environmental interactions between NOM and iron.
中文翻译:

铁(III)在作为天然有机物模型化合物的羧酸和酚存在下诱导的亚砷酸盐光氧化
铁物种对水和土壤中砷物种的环境/地球化学行为具有至关重要的影响。胶体氢氧化铁(CFH)通过表面络合和配体到金属的电荷转移(LMCT)诱导水中的砷氧化(As(III))氧化成砷(As(V))。然而,在此过程中,共存的天然有机物(NOM)对络合-光解的影响仍不清楚。在目前的工作中,研究了CFH诱导的As(III)的光氧化,它是作为NOM的简单模型化合物存在的各种羧酸和多酚的存在。紫外线A(UVA)(两个不同的光源λ最大 = 365纳米)和紫外线B(UVB)(λ最大 = 313nm)分别用于实验三元体系和对照二元体系的光氧化处理。获得的结果表明,所有研究的NOM都在pH值为7的条件下抑制了As(III)/ CFH体系中As(III)的光氧化作用。此外,准一级速率常数k obs与NOM的各种性质参数之间的相关性分析结果表明,Fe(III)与NOM的络合稳定常数(logK Fe-NOM)以及NOM的分子量和NOM的总酸度百分比显示出显着的相关性。建立了k obs之间的简单定量构效关系(QSAR)模型和这三个参数利用多元线性回归方法,可用于估计三价铁和三价铁存在下As(III)的光氧化效率。因此,目前的工作有助于理解NOM和铁之间的环境相互作用。













































 京公网安备 11010802027423号
京公网安备 11010802027423号