当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid Structure of Single and Mixed Cation Alkylammonium Bromide Urea Deep Eutectic Solvents.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.jpcb.0c06380
Manuel Brunner 1 , Silvia Imberti 2 , Gregory G Warr 3 , Rob Atkin 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.jpcb.0c06380
Manuel Brunner 1 , Silvia Imberti 2 , Gregory G Warr 3 , Rob Atkin 1
Affiliation
|
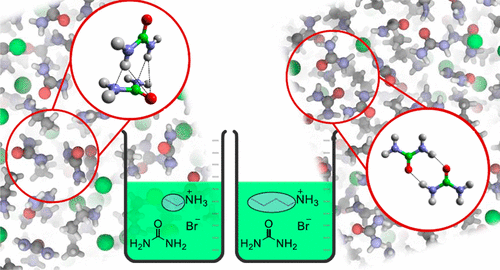
The liquid structures of three alkyl ammonium bromide and urea DESs, ethylammonium bromide:urea (1:1), butylammonium bromide:urea (1:1), and ethylammonium bromide/butylammonium bromide:urea (0.5:0.5:1), have been studied using small-angle neutron diffraction with H/D substituted sample contrasts. The diffraction data was fit using empirical potential structure refinement (EPSR). An amphiphilic nanostructure was found in all DESs due to cation alkyl chains being solvophobically excluded from charged domains, and due to clustering together. The polar domain was continuous in all three DESs, whereas the apolar domain was continuous for the butylammonium DES and in the mixed DES, but not the ethylammonium DES. This is attributed to solvophobic interactions being weaker for the short ethyl chain. Surprisingly, the urea also forms large clusters in all three DESs. In ethylammonium bromide:urea (1:1), urea–urea orientations are mainly perpendicular, but in butylammonium bromide:urea (1:1) and the mixed system in-plane and perpendicular arrangements are found. The liquid nanostructures found in this work, especially for the ethylammonium DES, are different from those found previously for the corresponding DESs formed using glycerol, revealing that the DES amphiphilic nanostructure is sensitive to the nature of the HBD (hydrogen bond donor).
中文翻译:

单和混合阳离子烷基溴化铵尿素深共晶溶剂的液体结构。
三种烷基溴化铵和脲DES,乙基溴化铵:脲(1:1),丁基溴化铵:脲(1:1)和乙基溴化铵/丁基溴化铵:脲(0.5:0.5:1)的液体结构使用小角度中子衍射和H / D替代样品对比进行研究。使用经验势能结构精修(EPSR)拟合衍射数据。在所有DES中都发现了两亲的纳米结构,这是由于阳离子烷基链被溶剂化地排除在带电域之外,并且由于聚集在一起。在所有三个DES中,极性结构域都是连续的,而对于丁基铵DES和混合的DES,非极性结构域是连续的,但是对于乙基铵DES,极性结构域是连续的。这归因于疏溶剂相互作用对短乙基链较弱。出奇,尿素还在所有三个DES中形成大簇。在溴化乙铵:尿素(1:1)中,尿素-尿素的取向主要是垂直的,而在溴化丁铵:尿素(1:1)中,则发现混合系统为面内和垂直排列。在这项工作中发现的液体纳米结构,特别是对于乙基铵DES,与之前发现的使用甘油形成的相应DES的液体纳米结构不同,这表明DES两亲纳米结构对HBD(氢键供体)的性质敏感。
更新日期:2020-10-02
中文翻译:

单和混合阳离子烷基溴化铵尿素深共晶溶剂的液体结构。
三种烷基溴化铵和脲DES,乙基溴化铵:脲(1:1),丁基溴化铵:脲(1:1)和乙基溴化铵/丁基溴化铵:脲(0.5:0.5:1)的液体结构使用小角度中子衍射和H / D替代样品对比进行研究。使用经验势能结构精修(EPSR)拟合衍射数据。在所有DES中都发现了两亲的纳米结构,这是由于阳离子烷基链被溶剂化地排除在带电域之外,并且由于聚集在一起。在所有三个DES中,极性结构域都是连续的,而对于丁基铵DES和混合的DES,非极性结构域是连续的,但是对于乙基铵DES,极性结构域是连续的。这归因于疏溶剂相互作用对短乙基链较弱。出奇,尿素还在所有三个DES中形成大簇。在溴化乙铵:尿素(1:1)中,尿素-尿素的取向主要是垂直的,而在溴化丁铵:尿素(1:1)中,则发现混合系统为面内和垂直排列。在这项工作中发现的液体纳米结构,特别是对于乙基铵DES,与之前发现的使用甘油形成的相应DES的液体纳米结构不同,这表明DES两亲纳米结构对HBD(氢键供体)的性质敏感。

































 京公网安备 11010802027423号
京公网安备 11010802027423号