当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytotoxic Screening and Transcriptomics Reveal Insights into the Molecular Mechanisms of Trihexyl Phosphate-Triggered Hepatotoxicity.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-08-25 , DOI: 10.1021/acs.est.0c03824 Zhenhua Li 1 , Xin Tang 1 , Lingfei Zhu 2 , Xiaojie Qi 1 , Gang Cao 2 , Gang Lu 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-08-25 , DOI: 10.1021/acs.est.0c03824 Zhenhua Li 1 , Xin Tang 1 , Lingfei Zhu 2 , Xiaojie Qi 1 , Gang Cao 2 , Gang Lu 2
Affiliation
|
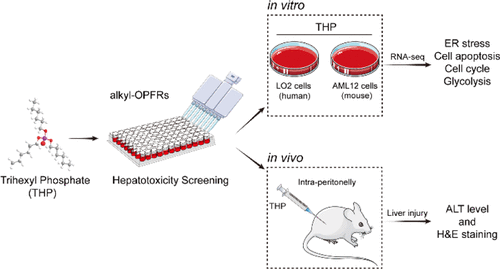
Mounting evidence shows that organophosphate flame retardants (OPFRs), especially aryl- and halogenated-OPFRs, exert various adverse health effects on living organisms. This study evaluated the hepatotoxic effect of trihexyl phosphate (THP) as a long-chain alkyl-OPFR on human hepatocyte cells (LO2) and mouse hepatocyte cells (AML12) by performing screening of cytotoxicity in vitro. In combination with transcriptomic analysis, toxicological mechanisms in vitro were further investigated. Results showed that THP triggered hepatotoxicity in vitro by altering four signaling pathways: endoplasmic reticulum (ER) stress, apoptosis, cell cycle, and the glycolysis signaling pathway. Exposure of LO2 and AML12 liver cells to THP (25 μg/mL) significantly induced ER stress-mediated apoptosis and cell cycle arrest. Meanwhile, downregulation of glycolysis caused the blockage of energy metabolism. Furthermore, the high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (HPLC-Q-TOF-MS/MS) revealed that much of THP was absorbed into the cells and displayed stability in the two liver cell lines. In vivo assays using a mouse model demonstrated that exposure to THP at 400 mg/kg induced the ballooning degeneration of hepatocytes in liver tissue, whereas exposure to THP at 800 mg/kg caused acute liver injury with high alanine aminotransferase levels. This study provides novel insights into the impact of THP on hepatotoxicity in vitro and in vivo and uncovers the underlying toxicological mechanisms, which may serve as a guide for further ecological risk assessment and reasonable application of alkyl-OPFRs.
中文翻译:

细胞毒性筛选和转录组学揭示了磷酸三己酯引发的肝毒性的分子机制。
越来越多的证据表明,有机磷酸酯阻燃剂(OPFR),尤其是芳基和卤代OPFR,会对生物体产生各种不利的健康影响。这项研究通过在体外进行细胞毒性筛选,评估了磷酸三己酯(THP)作为长链烷基-OPFR对人肝细胞(LO2)和小鼠肝细胞(AML12)的肝毒性作用。结合转录组学分析,进一步研究了体外毒理学机制。结果表明,THP通过改变四个信号传导途径触发了体外肝毒性:内质网应激,凋亡,细胞周期和糖酵解信号传导途径。LO2和AML12肝细胞暴露于THP(25μg/ mL)会显着诱导ER应激介导的细胞凋亡和细胞周期停滞。与此同时,糖酵解的下调导致能量代谢的障碍。此外,高效液相色谱-四极杆飞行时间质谱(HPLC-Q-TOF-MS / MS)显示,许多THP被细胞吸收并在两种肝细胞系中显示出稳定性。使用小鼠模型进行的体内分析表明,暴露于400 mg / kg的THP会诱导肝组织中肝细胞的气球状变性,而暴露于800 mg / kg的THP会导致丙氨酸转氨酶水平高的急性肝损伤。这项研究提供了关于THP在体外和体内对肝毒性影响的新颖见解,并揭示了潜在的毒理学机制,这可为进一步生态风险评估和合理应用烷基OPFRs提供指导。高效液相色谱-四极杆飞行时间质谱(HPLC-Q-TOF-MS / MS)显示,许多THP被细胞吸收并在两种肝细胞系中显示出稳定性。使用小鼠模型进行的体内分析表明,暴露于400 mg / kg的THP会诱导肝组织中肝细胞的气球状变性,而暴露于800 mg / kg的THP会导致丙氨酸氨基转移酶水平高的急性肝损伤。这项研究提供了关于THP在体外和体内对肝毒性影响的新颖见解,并揭示了潜在的毒理学机制,这可为进一步生态风险评估和合理应用烷基OPFRs提供指导。高效液相色谱-四极杆飞行时间质谱(HPLC-Q-TOF-MS / MS)显示,许多THP被细胞吸收并在两种肝细胞系中显示出稳定性。使用小鼠模型进行的体内分析表明,暴露于400 mg / kg的THP会诱导肝组织中肝细胞的气球状变性,而暴露于800 mg / kg的THP会导致丙氨酸转氨酶水平高的急性肝损伤。这项研究提供了关于THP在体外和体内对肝毒性影响的新颖见解,并揭示了潜在的毒理学机制,这可为进一步生态风险评估和合理应用烷基OPFRs提供指导。
更新日期:2020-09-15
中文翻译:

细胞毒性筛选和转录组学揭示了磷酸三己酯引发的肝毒性的分子机制。
越来越多的证据表明,有机磷酸酯阻燃剂(OPFR),尤其是芳基和卤代OPFR,会对生物体产生各种不利的健康影响。这项研究通过在体外进行细胞毒性筛选,评估了磷酸三己酯(THP)作为长链烷基-OPFR对人肝细胞(LO2)和小鼠肝细胞(AML12)的肝毒性作用。结合转录组学分析,进一步研究了体外毒理学机制。结果表明,THP通过改变四个信号传导途径触发了体外肝毒性:内质网应激,凋亡,细胞周期和糖酵解信号传导途径。LO2和AML12肝细胞暴露于THP(25μg/ mL)会显着诱导ER应激介导的细胞凋亡和细胞周期停滞。与此同时,糖酵解的下调导致能量代谢的障碍。此外,高效液相色谱-四极杆飞行时间质谱(HPLC-Q-TOF-MS / MS)显示,许多THP被细胞吸收并在两种肝细胞系中显示出稳定性。使用小鼠模型进行的体内分析表明,暴露于400 mg / kg的THP会诱导肝组织中肝细胞的气球状变性,而暴露于800 mg / kg的THP会导致丙氨酸转氨酶水平高的急性肝损伤。这项研究提供了关于THP在体外和体内对肝毒性影响的新颖见解,并揭示了潜在的毒理学机制,这可为进一步生态风险评估和合理应用烷基OPFRs提供指导。高效液相色谱-四极杆飞行时间质谱(HPLC-Q-TOF-MS / MS)显示,许多THP被细胞吸收并在两种肝细胞系中显示出稳定性。使用小鼠模型进行的体内分析表明,暴露于400 mg / kg的THP会诱导肝组织中肝细胞的气球状变性,而暴露于800 mg / kg的THP会导致丙氨酸氨基转移酶水平高的急性肝损伤。这项研究提供了关于THP在体外和体内对肝毒性影响的新颖见解,并揭示了潜在的毒理学机制,这可为进一步生态风险评估和合理应用烷基OPFRs提供指导。高效液相色谱-四极杆飞行时间质谱(HPLC-Q-TOF-MS / MS)显示,许多THP被细胞吸收并在两种肝细胞系中显示出稳定性。使用小鼠模型进行的体内分析表明,暴露于400 mg / kg的THP会诱导肝组织中肝细胞的气球状变性,而暴露于800 mg / kg的THP会导致丙氨酸转氨酶水平高的急性肝损伤。这项研究提供了关于THP在体外和体内对肝毒性影响的新颖见解,并揭示了潜在的毒理学机制,这可为进一步生态风险评估和合理应用烷基OPFRs提供指导。































 京公网安备 11010802027423号
京公网安备 11010802027423号