Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Insights into the Structure and Reactivity of Uracil Derivatives in Different Solvents-A Computational Study.
ACS Omega ( IF 3.7 ) Pub Date : 2020-08-24 , DOI: 10.1021/acsomega.0c02943 Shahidul M Islam 1 , Zahin Ibnat 1
ACS Omega ( IF 3.7 ) Pub Date : 2020-08-24 , DOI: 10.1021/acsomega.0c02943 Shahidul M Islam 1 , Zahin Ibnat 1
Affiliation
|
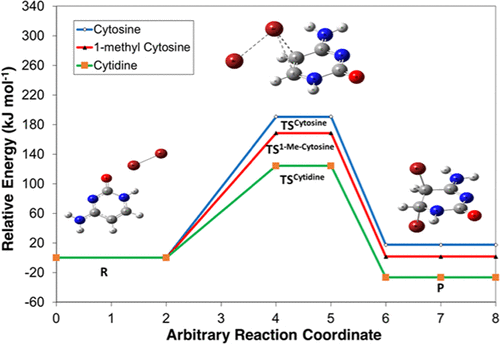
Ab initio calculations were carried out to understand the reactivity and stability of some uracil derivatives, cytosine, 1-methyl cytosine, and cytidine in solvents, water, dimethyl sulfoxide (DMSO), n-octanol, and chloroform. Geometries were fully optimized at MP2 and B3LYP using the 6-31+G(d,p) basis set by applying the Solvation Model on Density (SMD) in solvent systems. The syn conformer of cytidine (cytidine II) is the most stable conformer in the gas phase, while the anticonformer (cytidine IV) is most stable in all of the solvents. Solvation free energy and polarizability values in different solvents decrease in the order water > DMSO > n-octanol > chloroform, while dipole moment, first-order hyperpolarizability, and HOMO–LUMO energy gap values follow the order of polar protic solvent (water and n-octanol) > polar aprotic solvent (DMSO) > nonpolar solvent (chloroform). The solvation free energy, dipole moment, polarizability, and first-order hyperpolarizability values also follow the order of cytosine > 1-methyl cytosine > cytidine. To illustrate that the molecular properties correlate well with the reactivity of the molecules, ab initio calculations were carried out for the reaction of uracil derivatives with Br2 in the gas phase, water, DMSO, n-octanol, and chloroform. All ground and transition state geometries were fully optimized at B3LYP/6-31+G(d,p), and energies were also calculated at G3MP2 for cytosine and 1-methyl cytosine. For cytosine and 1-methyl cytosine, Gibbs energies of activation decrease with the polarity of the solvent that is chloroform > n-octanol > DMSO > water, while the Gibbs energies of activation for the reaction with cytidine decrease in the order of water > DMSO > n-octanol > chloroform. These results suggest that solvent polarity is very important for the stability and reactivity of uracil derivatives. Hydrogen bonding may also play an important role mainly for cytidine. Free energies of activation decrease with the size of the molecule, i.e., cytosine > 1-methyl cytosine > cytidine.
中文翻译:

对尿嘧啶衍生物在不同溶剂中的结构和反应性的新见解——计算研究。
进行从头计算以了解一些尿嘧啶衍生物、胞嘧啶、1-甲基胞嘧啶和胞苷在溶剂、水、二甲亚砜 (DMSO)、正辛醇和氯仿中的反应性和稳定性。通过在溶剂系统中应用密度溶剂化模型 (SMD),使用 6-31+G(d,p) 基组在 MP2 和 B3LYP 上全面优化几何形状。胞苷的顺式构象异构体(胞苷II)是气相中最稳定的构象异构体,而反构象异构体(胞苷IV)在所有溶剂中是最稳定的。不同溶剂中的溶剂化自由能和极化率值按水> DMSO>正辛醇>氯仿的顺序递减,而偶极矩、一阶超极化率和HOMO-LUMO能隙值遵循极性质子溶剂的顺序(水和正辛醇)%3E极性非质子溶剂(DMSO)%3E非极性溶剂(氯仿)。溶剂化自由能、偶极矩、极化率和一级超极化率值也遵循胞嘧啶> 1-甲基胞嘧啶> 胞苷的顺序。为了说明分子性质与分子的反应性良好相关,对尿嘧啶衍生物与Br 2在气相、水、DMSO、正辛醇和氯仿中的反应进行了从头算。所有基态和过渡态几何形状均在 B3LYP/6-31+G(d,p) 上进行了全面优化,并且还在 G3MP2 上计算了胞嘧啶和 1-甲基胞嘧啶的能量。 对于胞嘧啶和1-甲基胞嘧啶,吉布斯活化能随着溶剂氯仿>正辛醇> DMSO>水的极性而降低,而与胞苷反应的吉布斯活化能按以下顺序降低:水 > DMSO >正辛醇 > 氯仿。这些结果表明溶剂极性对于尿嘧啶衍生物的稳定性和反应性非常重要。氢键也可能主要对于胞苷发挥重要作用。活化自由能随分子大小而降低,即胞嘧啶> 1-甲基胞嘧啶> 胞苷。
更新日期:2020-09-08
中文翻译:

对尿嘧啶衍生物在不同溶剂中的结构和反应性的新见解——计算研究。
进行从头计算以了解一些尿嘧啶衍生物、胞嘧啶、1-甲基胞嘧啶和胞苷在溶剂、水、二甲亚砜 (DMSO)、正辛醇和氯仿中的反应性和稳定性。通过在溶剂系统中应用密度溶剂化模型 (SMD),使用 6-31+G(d,p) 基组在 MP2 和 B3LYP 上全面优化几何形状。胞苷的顺式构象异构体(胞苷II)是气相中最稳定的构象异构体,而反构象异构体(胞苷IV)在所有溶剂中是最稳定的。不同溶剂中的溶剂化自由能和极化率值按水> DMSO>正辛醇>氯仿的顺序递减,而偶极矩、一阶超极化率和HOMO-LUMO能隙值遵循极性质子溶剂的顺序(水和正辛醇)%3E极性非质子溶剂(DMSO)%3E非极性溶剂(氯仿)。溶剂化自由能、偶极矩、极化率和一级超极化率值也遵循胞嘧啶> 1-甲基胞嘧啶> 胞苷的顺序。为了说明分子性质与分子的反应性良好相关,对尿嘧啶衍生物与Br 2在气相、水、DMSO、正辛醇和氯仿中的反应进行了从头算。所有基态和过渡态几何形状均在 B3LYP/6-31+G(d,p) 上进行了全面优化,并且还在 G3MP2 上计算了胞嘧啶和 1-甲基胞嘧啶的能量。 对于胞嘧啶和1-甲基胞嘧啶,吉布斯活化能随着溶剂氯仿>正辛醇> DMSO>水的极性而降低,而与胞苷反应的吉布斯活化能按以下顺序降低:水 > DMSO >正辛醇 > 氯仿。这些结果表明溶剂极性对于尿嘧啶衍生物的稳定性和反应性非常重要。氢键也可能主要对于胞苷发挥重要作用。活化自由能随分子大小而降低,即胞嘧啶> 1-甲基胞嘧啶> 胞苷。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号