Journal of Advanced Research ( IF 11.4 ) Pub Date : 2020-08-24 , DOI: 10.1016/j.jare.2020.08.011 Sunli Hu 1, 2, 3 , Liang Chen 1, 2, 3 , Abdullah Al Mamun 4 , Libin Ni 1, 2, 3 , Weiyang Gao 1 , Yan Lin 1 , Haiming Jin 1 , Xiaolei Zhang 1, 2 , Xiangyang Wang 1, 3
|
Introduction
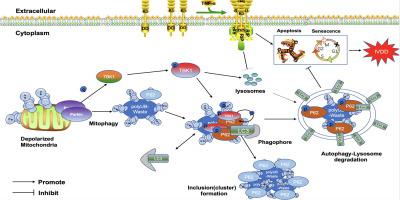
While its innate immune function has been known, recent works of literature have focused on the role of Tank binding kinase 1 (TBK1) in regulating autophagy and it is unknown whether TBK1 protects against intervertebral disc degeneration (IVDD) through affecting autophagy.
Objectives
Here, we aim to explore whether TBK1 is implicated in the pathogenesis of IVDD, and investigated the potential mechanism.
Methods
Western blotting and immunohistochemistry were used to detect the TBK1 expression in human and rat NP tissue. After TBK1 overexpression in NP cells with lentivirus transfection, autophagic flux, apoptosis and senescence percentage were assessed. Si-RNA , a utophagy inhibitors and protein phosphatase inhibitors were applied to study the mechanism of autophagy regulation. In vivo study, we further evaluated the therapeutic action of lentivirus-TBK1(Lv-TBK1)injection in a rodent IVDD model.
Results
The TBK1 level was reduced in rat and human NP tissue. TBK1 overexpression protected against apoptosis and premature senescence. These functions of TBK1 were abolished by chloroquine-medicated autophagy inhibition.P-TBK1, an activation form of TBK, is involved in selective autophagy through directly phosphorylating P62 at Ser 403, and the activation of TBK1 is also dependent on Parkin manner. TBK1 also activated NPCs autophagy to relieve puncture injury in vivo.
Conclusion
We demonstrated that TBK1 overexpression attenuated senescence and apoptosis and promoted NPCs survival via upregulating autophagy. TBK1 represents a promising avenue for IVDD treatment.
中文翻译:

TBK1通过协调选择性自噬和自噬功能治疗椎间盘退变的疗效
介绍
虽然其先天免疫功能已为人所知,但最近的文献工作集中在坦克结合激酶 1 (TBK1) 在调节自噬中的作用,尚不清楚 TBK1 是否通过影响自噬来防止椎间盘退变 (IVDD)。
目标
在这里,我们旨在探讨 TBK1 是否与 IVDD 的发病机制有关,并研究其潜在机制。
方法
Western印迹和免疫组织化学用于检测人和大鼠NP组织中TBK1的表达。在慢病毒转染的 NP 细胞中 TBK1 过表达后,评估了自噬通量、细胞凋亡和衰老百分比。应用Si-RNA、自噬抑制剂和蛋白磷酸酶抑制剂研究自噬调控机制。在体内研究中,我们进一步评估了慢病毒-TBK1(Lv-TBK1)注射液在啮齿动物 IVDD 模型中的治疗作用。
结果
大鼠和人 NP 组织中的 TBK1 水平降低。TBK1 过表达可防止细胞凋亡和过早衰老。TBK1 的这些功能被氯喹介导的自噬抑制所消除。 P-TBK1 是 TBK 的一种激活形式,通过直接在 Ser 403 磷酸化 P62 参与选择性自噬,并且 TBK1 的激活也依赖于 Parkin 方式。TBK1 还激活 NPCs 自噬以减轻体内穿刺损伤。
结论
我们证明了 TBK1 过表达减弱了衰老和细胞凋亡,并通过上调自噬促进了 NPC 的存活。TBK1 代表了 IVDD 治疗的一个有前途的途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号