European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-08-22 , DOI: 10.1016/j.ejmech.2020.112774 Han Zhang 1 , Xiaomeng He 1 , Xintong Wang 2 , Bo Yu 1 , Siqi Zhao 1 , Peili Jiao 1 , Hongwei Jin 1 , Zhenming Liu 1 , KeWei Wang 3 , Liangren Zhang 1 , Lihe Zhang 1
|
α
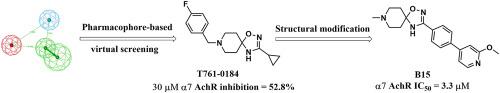
7 nicotinic acetylcholine receptors (nAChRs) expressed in the nervous and immune systems have been suggested to play important roles in the control of inflammation. However, the lack of antagonist tools specifically inhibiting α7 nAChR impedes the validation of the channel as therapeutic target. To discover a selective α7 antagonist, we started a pharmacophore-based virtual screening and identified a piperidine-spirooxadiazole derivative T761–0184 that acts as a α7 antagonist. A series of novel piperidine-spirooxadiazole derivatives were subsequently synthesized and evaluated using two-electrode voltage clamp (TEVC) assay in Xenopus oocytes. Lead compounds from two series inhibited α7 with their IC50 values ranging from 3.3 μM to 13.7 μM. Compound B10 exhibited α7 selectivity over other α4β2 and α3β4 nAChR subtypes. The analysis of structure-activity relationship (SAR) provides valuable insights for further development of selective α7 nAChR antagonists.
中文翻译:

哌啶-螺恶二唑衍生物作为α7烟碱样受体拮抗剂的设计,合成和生物学活性。
α
已经提出在神经和免疫系统中表达的7种烟碱乙酰胆碱受体(nAChRs)在炎症控制中起重要作用。然而,由于缺乏的拮抗剂工具特异性抑制α 7个的nAChR阻碍通道作为治疗靶的验证。要发现的选择性α 7拮抗剂,我们开始了基于药效的虚拟筛选和鉴定的哌啶衍生物spirooxadiazole T761 - 0184充当α 7拮抗剂。随后在非洲爪蟾卵母细胞中合成了一系列新型哌啶-螺二恶唑衍生物,并使用两电极电压钳(TEVC)分析对其进行了评估。两个系列的铅化合物均受抑制α7的IC 50值为3.3μM至13.7μM。化合物B10表现出α 7选择性超过其它α 4 β 2和α 3 β 4的nAChR亚型。的结构-活性关系的分析(SAR)提供了一种用于选择性的进一步发展了有价值的见解α 7的nAChR拮抗剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号