Chemosphere ( IF 8.1 ) Pub Date : 2020-08-23 , DOI: 10.1016/j.chemosphere.2020.128067 Haopeng Luo , Xin Zhou , Xiaojie Guo , Zhiyong Fang , Quanyuan Chen , Juan Zhou
|
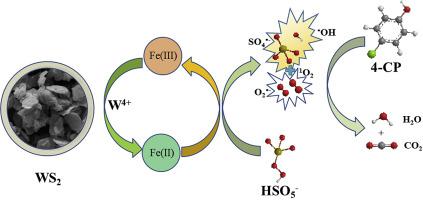
The greatest constraint in the advanced oxidation processes involved Fe(II)/PMS was the low utilization of Fe(II) and PMS. In the present study, the co-catalytic effect of WS2 on the Fe(II)/PMS system for the degradation of organics was investigated. In the presence of WS2, Fe(III) was reduced to Fe(II) during the reaction and resulted in improved decomposition of PMS as well as the degradation of 4-chloriphenol (4-CP). The decomposition rate of PMS and degradation efficiency of 4-CP were 10% and 25% in the Fe(II)/PMS process, while the efficiencies respectively increased to 99% and 100% in the WS2 assisted Fe(II)/PMS system. The degradation of 4-CP was completed via the free radical pathway and SO4•- played a more important role than other active species. Low concentration of inorganic ions such as Cl- and HCO3- exhibited irrelevant effect while humic acid showed significant suppression on the WS2/Fe(II)/PMS system. Additionally, characterization and recycle results implied that WS2 maintained a good stability during the co-catalytic processes.
中文翻译:

WS 2作为高级氧化过程中Fe(II)再生的高活性助催化剂
在涉及Fe(II)/ PMS的高级氧化工艺中,最大的制约因素是Fe(II)和PMS的利用率低。在本研究中,研究了WS 2对Fe(II)/ PMS系统对有机物降解的共催化作用。在WS 2的存在下,Fe(III)在反应过程中还原为Fe(II),导致PMS的分解得到改善,4-氯苯酚(4-CP)降解。Fe(II)/ PMS工艺中PMS的分解率和4-CP的降解效率分别为10%和25%,而WS 2辅助Fe(II)/ PMS中PMS的分解效率分别为99%和100%系统。4-CP的降解通过自由基途径和SO 4 • -完成。发挥了比其他活跃物种更重要的作用。无机离子如Cl的低浓度-和HCO 3 - ,而腐殖酸显示对WS显著抑制发挥效果无关2 /铁(II)/ PMS系统。此外,表征和再循环结果表明WS 2在共催化过程中保持了良好的稳定性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号