当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereochemical Inversion of Rim-Differentiated Pillar[5]arene Molecular Swings.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.joc.0c01464 Ke Du 1 , Paul Demay-Drouhard 1, 2 , Kushal Samanta 1, 2 , Shunshun Li 1 , Tushar Ulhas Thikekar 1 , Haiying Wang 1 , Minjie Guo 1 , Barend van Lagen 2 , Han Zuilhof 1, 2, 3 , Andrew C-H Sue 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.joc.0c01464 Ke Du 1 , Paul Demay-Drouhard 1, 2 , Kushal Samanta 1, 2 , Shunshun Li 1 , Tushar Ulhas Thikekar 1 , Haiying Wang 1 , Minjie Guo 1 , Barend van Lagen 2 , Han Zuilhof 1, 2, 3 , Andrew C-H Sue 1
Affiliation
|
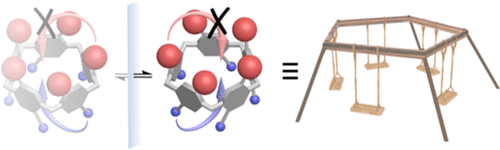
To investigate the dynamic stereochemical inversion behavior of pillar[5]arenes (P[5]s) in more detail, we synthesized a series of novel rim-differentiated P[5]s with various substituents and examined their rapid rotations by variable-temperature NMR (203–298 K). These studies revealed for the first time the barrier of “methyl-through-the-annulus” rotation (ΔG‡ = 47.4 kJ·mol–1 in acetone) and indicated that for rim-differentiated P[5]s with two types of alkyl substituents, the smaller rim typically determines the rate of rotation. However, substituents with terminal C═C or C≡C bonds give rise to lower inversion barriers, presumably as a result of attractive π–π interactions in the transition state. Finally, data on a rim-differentiated penta-methyl-penta-propargyl P[5] exhibited the complexity of the overall inversion dynamics.
中文翻译:

Rim差异柱[5]芳烃分子摇摆的立体化学反演。
为了更详细地研究支柱[5]芳烃(P [5] s)的动态立体化学反转行为,我们合成了一系列具有各种取代基的新型边缘分化P [5] s,并通过可变温度检查了它们的快速旋转NMR(203–298 K)。这些研究首次揭示了“甲基环空”旋转的障碍(ΔG ‡ = 47.4 kJ·mol –1并指出对于具有两种类型烷基取代基的边缘差异化P [5],较小的边缘通常决定旋转速率。但是,带有末端C═C或C≡C键的取代基会导致较低的反型垒,这大概是由于过渡态π-π相互作用引起的。最后,关于边缘分化的五甲基五戊炔丙基P [5]的数据显示了整体反演动力学的复杂性。
更新日期:2020-09-05
中文翻译:

Rim差异柱[5]芳烃分子摇摆的立体化学反演。
为了更详细地研究支柱[5]芳烃(P [5] s)的动态立体化学反转行为,我们合成了一系列具有各种取代基的新型边缘分化P [5] s,并通过可变温度检查了它们的快速旋转NMR(203–298 K)。这些研究首次揭示了“甲基环空”旋转的障碍(ΔG ‡ = 47.4 kJ·mol –1并指出对于具有两种类型烷基取代基的边缘差异化P [5],较小的边缘通常决定旋转速率。但是,带有末端C═C或C≡C键的取代基会导致较低的反型垒,这大概是由于过渡态π-π相互作用引起的。最后,关于边缘分化的五甲基五戊炔丙基P [5]的数据显示了整体反演动力学的复杂性。































 京公网安备 11010802027423号
京公网安备 11010802027423号