当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
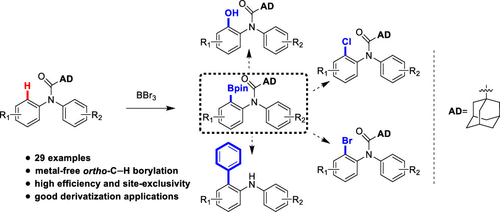
C-H Borylation of Diphenylamines through Adamantane-1-carbonyl Auxiliary by BBr3.
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.orglett.0c02552 Gaorong Wu 1 , Xiaopan Fu 1 , Yangyang Wang 1 , Kezuan Deng 1 , Lili Zhang 1 , Tao Ma 2 , Yafei Ji 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.orglett.0c02552 Gaorong Wu 1 , Xiaopan Fu 1 , Yangyang Wang 1 , Kezuan Deng 1 , Lili Zhang 1 , Tao Ma 2 , Yafei Ji 1
Affiliation
|
A method for ortho-C–H borylation of diphenylamines using BBr3 as the boron source has been reported. The noncatalytic adamantane-1-carbonyl directed reaction exhibited site exclusivity and good functional group tolerance. Generally, the borylation occurred at the more electron-rich aromatic ring and the borylated products could be converted to various useful intermediates. Besides, the derived arylation and removal of auxiliary of the product could be achieved in a one-pot fashion.
中文翻译:

BBr3通过金刚烷-1-羰基辅助剂将二苯胺的CH硼化反应。
已经报道了一种使用BBr 3作为硼源的邻苯二甲胺的CH-H硼酸酯化方法。非催化金刚烷-1-羰基定向反应具有位点排他性和良好的官能团耐受性。通常,硼化发生在较富电子的芳环上,并且硼化的产物可以转化为各种有用的中间体。此外,可以通过一锅法实现衍生的芳基化和产物助剂的去除。
更新日期:2020-09-05
中文翻译:

BBr3通过金刚烷-1-羰基辅助剂将二苯胺的CH硼化反应。
已经报道了一种使用BBr 3作为硼源的邻苯二甲胺的CH-H硼酸酯化方法。非催化金刚烷-1-羰基定向反应具有位点排他性和良好的官能团耐受性。通常,硼化发生在较富电子的芳环上,并且硼化的产物可以转化为各种有用的中间体。此外,可以通过一锅法实现衍生的芳基化和产物助剂的去除。











































 京公网安备 11010802027423号
京公网安备 11010802027423号