当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of N-Arylsulfonyl-Indole-2-Carboxamide Derivatives as Potent, Selective, and Orally Bioavailable Fructose-1,6-Bisphosphatase Inhibitors-Design, Synthesis, In Vivo Glucose Lowering Effects, and X-ray Crystal Complex Analysis.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.jmedchem.0c00726 Jie Zhou 1 , Jianbo Bie 1 , Xiaoyu Wang 1 , Quan Liu 2 , Rongcui Li 2 , Hualong Chen 1 , Jinping Hu 3 , Hui Cao 2 , Wenming Ji 2 , Yan Li 3 , Shuainan Liu 2 , Zhufang Shen 2 , Bailing Xu 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.jmedchem.0c00726 Jie Zhou 1 , Jianbo Bie 1 , Xiaoyu Wang 1 , Quan Liu 2 , Rongcui Li 2 , Hualong Chen 1 , Jinping Hu 3 , Hui Cao 2 , Wenming Ji 2 , Yan Li 3 , Shuainan Liu 2 , Zhufang Shen 2 , Bailing Xu 1
Affiliation
|
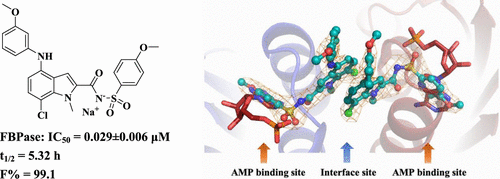
Liver fructose-1,6-bisphosphatase (FBPase) is a key enzyme in the gluconeogenesis pathway. Inhibiting FBPase activity represents a potential treatment for type 2 diabetes mellitus. A series of novel N-arylsulfonyl-4-arylamino-indole-2-carboxamide derivatives have been disclosed as FBPase inhibitors. Through extensive structure–activity relationship investigations, a promising candidate molecule Cpd118 [sodium (7-chloro-4-((3-methoxyphenyl)amino)-1-methyl-1H-indole-2-carbonyl] [(4-methoxyphenyl)sulfonyl)amide] has been identified with high inhibitory activity against human liver FBPase (IC50, 0.029 ± 0.006 μM) and high selectivity relative to the other six AMP-binding enzymes. Importantly, Cpd118 produced significant glucose-lowering effects on both type 2 diabetic KKAy mice and ZDF rats as demonstrated by substantial reductions in the fasting and postprandial blood glucose levels, as well as the HbA1c level. Furthermore, Cpd118 elicited a favorable pharmacokinetic profile with an oral bioavailability of 99.1%. Moreover, the X-ray crystal structure of the Cpd118–FBPase complex was resolved, which revealed a unique binding mode and provided a structural basis for its high potency and selectivity.
中文翻译:

发现作为有效的,选择性的和口服生物利用的果糖1,6,6-双磷酸酶抑制剂的N-芳基磺酰基-吲哚-2-羧酰胺衍生物-设计,合成,体内葡萄糖降低作用和X射线晶体复合物分析。
肝果糖-1,6-双磷酸酶(FBPase)是糖异生途径中的关键酶。抑制FBPase活性代表2型糖尿病的潜在治疗方法。已经公开了一系列新颖的N-芳基磺酰基-4-芳基氨基-吲哚-2-羧酰胺衍生物作为FBPase抑制剂。通过广泛的结构-活性关系研究,一个有前途的候选分子Cpd118 [(7-氯-4-((3-甲氧基苯基)氨基)-1-甲基-1 H-吲哚-2-羰基钠] [(4-甲氧基苯基)相对于其他六种AMP结合酶,已鉴定出对人肝FBPase的抑制活性高(IC 50为0.029±0.006μM),并且具有较高的选择性。重要的是,Cpd118空腹和餐后血糖水平以及HbA1c水平明显降低,证明2型糖尿病KKAy小鼠和ZDF大鼠均具有明显的降糖作用。此外,Cpd118引起了良好的药代动力学特征,口服生物利用度为99.1%。此外,解析了Cpd118 -FBPase复合物的X射线晶体结构,揭示了独特的结合模式,并为其高效力和选择性提供了结构基础。
更新日期:2020-09-24
中文翻译:

发现作为有效的,选择性的和口服生物利用的果糖1,6,6-双磷酸酶抑制剂的N-芳基磺酰基-吲哚-2-羧酰胺衍生物-设计,合成,体内葡萄糖降低作用和X射线晶体复合物分析。
肝果糖-1,6-双磷酸酶(FBPase)是糖异生途径中的关键酶。抑制FBPase活性代表2型糖尿病的潜在治疗方法。已经公开了一系列新颖的N-芳基磺酰基-4-芳基氨基-吲哚-2-羧酰胺衍生物作为FBPase抑制剂。通过广泛的结构-活性关系研究,一个有前途的候选分子Cpd118 [(7-氯-4-((3-甲氧基苯基)氨基)-1-甲基-1 H-吲哚-2-羰基钠] [(4-甲氧基苯基)相对于其他六种AMP结合酶,已鉴定出对人肝FBPase的抑制活性高(IC 50为0.029±0.006μM),并且具有较高的选择性。重要的是,Cpd118空腹和餐后血糖水平以及HbA1c水平明显降低,证明2型糖尿病KKAy小鼠和ZDF大鼠均具有明显的降糖作用。此外,Cpd118引起了良好的药代动力学特征,口服生物利用度为99.1%。此外,解析了Cpd118 -FBPase复合物的X射线晶体结构,揭示了独特的结合模式,并为其高效力和选择性提供了结构基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号