当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual Effect of the Acidic Polysaccharose Ulvan on the Inhibition of Amyloid-β Protein Fibrillation and Disintegration of Mature Fibrils.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-08-20 , DOI: 10.1021/acsami.0c14292 Fufeng Liu 1, 2, 3 , Wenping Zhao 3 , Fang Zhao 3 , Qinchen Dong 3 , Ying Wang 3 , Wei Wei 3 , Longgang Jia 4 , Li Li 5 , Fuping Lu 1, 2, 3
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-08-20 , DOI: 10.1021/acsami.0c14292 Fufeng Liu 1, 2, 3 , Wenping Zhao 3 , Fang Zhao 3 , Qinchen Dong 3 , Ying Wang 3 , Wei Wei 3 , Longgang Jia 4 , Li Li 5 , Fuping Lu 1, 2, 3
Affiliation
|
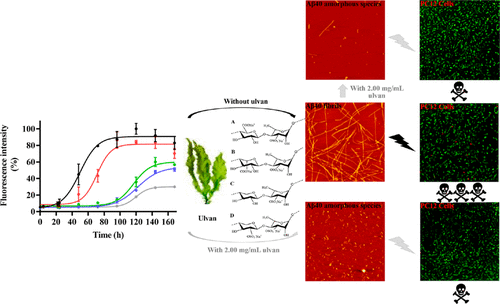
The abnormal folding and aggregation of amyloid-β protein (Aβ) is the main reason for the occurrence and development of Alzheimer’s disease (AD). The discovery of novel inhibitors against Aβ aggregation is still the current research focus. Herein, we report the inhibitory effect of ulvan, an acidic polysaccharide from green algae of the genus Ulva, against Aβ fibrillation using thioflavin T (ThT) fluorescence and atomic force microscopy (AFM) assays. It is shown that ulvan effectively inhibits Aβ fibrillogenesis in a concentration-dependent manner and actively inhibits the formation of A11-reactive Aβ oligomers, the most toxic Aβ species. The circular dichroism spectrum reveals that ulvan blocks the conformational transition of Aβ40 from the initial random coil to a β-sheet structure, but it only delays the conformational transition of Aβ42. It is also found that ulvan greatly reduces Aβ-induced cytotoxicity by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In addition, ulvan effectively downregulates intracellular reactive oxygen species production and protects PC12 cells from the damage caused by Aβ fibrillation. Moreover, ulvan disaggregates preformed mature fibrils into off-pathway oligomers and greatly decreases their associated cytotoxicity, as revealed using ThT fluorescence, AFM, MTT, and dot-blotting assays. The above results not only fully describe the inhibitory effect of ulvan on Aβ fibrillation and its related cytotoxicity but also provide novel ideas for the development of functional food ingredients from seaweed to treat AD.
中文翻译:

酸性多糖Ulvan对抑制淀粉样β蛋白原纤化和成熟原纤维分解的双重作用。
淀粉样β蛋白(Aβ)的异常折叠和聚集是阿尔茨海默氏病(AD)发生和发展的主要原因。新型抗Aβ聚集抑制剂的发现仍然是当前的研究重点。在此,我们报道了ulvan(一种来自Ulva属绿藻的酸性多糖)的抑制作用,通过使用硫代黄素T(ThT)荧光和原子力显微镜(AFM)分析来抗Aβ纤颤。结果表明,ulvan以浓度依赖的方式有效抑制Aβ原纤维形成,并积极抑制A11反应性Aβ低聚物(毒性最高的Aβ物种)的形成。圆二色性光谱表明,ulvan阻止了Aβ40从初始无规卷曲到β-折叠结构的构象转变,但仅延迟了Aβ42的构象转变。还发现ulvan通过3-(4,5-二甲基噻唑-2-基)-2,5-二苯基四唑溴化物(MTT)测定大大降低了Aβ诱导的细胞毒性。此外,ulvan有效地下调细胞内活性氧的产生并保护PC12细胞免受Aβ纤颤引起的损害。此外,如ThT荧光,AFM,MTT和斑点印迹分析所揭示的那样,ulvan将预先形成的成熟原纤维分解为非通路寡聚物,并大大降低了其相关的细胞毒性。上述结果不仅充分描述了ulvan对Aβ原纤维形成的抑制作用及其相关的细胞毒性,而且为开发从海藻中治疗AD的功能性食品成分提供了新思路。
更新日期:2020-09-16
中文翻译:

酸性多糖Ulvan对抑制淀粉样β蛋白原纤化和成熟原纤维分解的双重作用。
淀粉样β蛋白(Aβ)的异常折叠和聚集是阿尔茨海默氏病(AD)发生和发展的主要原因。新型抗Aβ聚集抑制剂的发现仍然是当前的研究重点。在此,我们报道了ulvan(一种来自Ulva属绿藻的酸性多糖)的抑制作用,通过使用硫代黄素T(ThT)荧光和原子力显微镜(AFM)分析来抗Aβ纤颤。结果表明,ulvan以浓度依赖的方式有效抑制Aβ原纤维形成,并积极抑制A11反应性Aβ低聚物(毒性最高的Aβ物种)的形成。圆二色性光谱表明,ulvan阻止了Aβ40从初始无规卷曲到β-折叠结构的构象转变,但仅延迟了Aβ42的构象转变。还发现ulvan通过3-(4,5-二甲基噻唑-2-基)-2,5-二苯基四唑溴化物(MTT)测定大大降低了Aβ诱导的细胞毒性。此外,ulvan有效地下调细胞内活性氧的产生并保护PC12细胞免受Aβ纤颤引起的损害。此外,如ThT荧光,AFM,MTT和斑点印迹分析所揭示的那样,ulvan将预先形成的成熟原纤维分解为非通路寡聚物,并大大降低了其相关的细胞毒性。上述结果不仅充分描述了ulvan对Aβ原纤维形成的抑制作用及其相关的细胞毒性,而且为开发从海藻中治疗AD的功能性食品成分提供了新思路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号