当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nano PdFe Alloy Assembled Film as a Highly Efficient Electrocatalyst toward Hydrogen Evolution in Both Acid and Alkaline Solutions
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-08-20 , DOI: 10.1021/acsaem.0c01410 Hanruo Chen 1 , Min Yuan 1 , Conghui Zhai 1 , Lingjun Tan 1 , Ning Cong 1 , Juanjuan Han 1 , Hua Fang 1 , Xiaorong Zhou 1 , Zhandong Ren 1 , Yuchan Zhu 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-08-20 , DOI: 10.1021/acsaem.0c01410 Hanruo Chen 1 , Min Yuan 1 , Conghui Zhai 1 , Lingjun Tan 1 , Ning Cong 1 , Juanjuan Han 1 , Hua Fang 1 , Xiaorong Zhou 1 , Zhandong Ren 1 , Yuchan Zhu 1
Affiliation
|
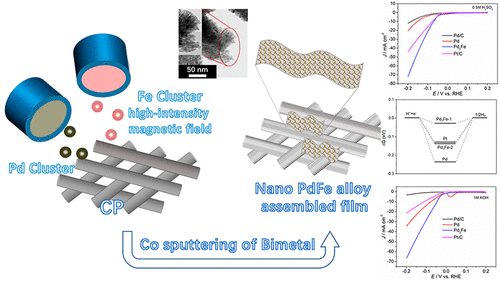
A series of nano PdFe alloy film electrodes with different ratios were prepared by high-vacuum magnetron sputtering under the action of high-strength magnetic field. Among them, the Pd3Fe alloy film electrode possesses the best hydrogen evolution activity, which significantly changes the properties of hydrogen adsorption (Had) and absorption (Habs) of Pd. Pd3Fe is a nanoassembled film, which consists of many tiny nanosheets connected together. The electrochemical surface area of Pd3Fe electrode is 13 and 7 times that of the Pd/C and Pd electrode, respectively. At the same time, Pd and Fe had formed an alloy structure. The (111) plane of the Pd3Fe alloy is an obvious positive shift, and the crystal cell is compressed. The electron is transferred from Fe to Pd through XPS analysis, which has proven the electronic relationship between Pd and Fe. DFT calculations have also shown that the ΔGH* of the Pd3Fe surface is significantly reduced compared to that of Pd. Reducing the desorption energy of H on the Pd surface is beneficial to improve the electrocatalytic performance. It is noteworthy that the HER activity of Pd3Fe electrode has exceeded that of Pd/C, Pd, and Pt/C in both 0.5 M H2SO4 and 1.0 M KOH. The HER activity of Pd3Fe electrode is 20, 1.9, and 3.1 times that of Pd/C, Pd, and Pt/C in 1.0 M KOH at −0.2 V. In 0.5 M H2SO4, the HER activity of the Pd3Fe electrode is 6, 3.5, and 1.6 times that of Pd/C, Pd, and Pt/C. The Pd3Fe film electrode has the most favorable reaction kinetics, with Tafel slopes of 44.7 and 38.7 mV dec–1 in acidic and alkaline solutions, respectively. The values of the Tafel slope indicate that the mechanism of HER on the Pd3Fe film electrode is consistent with the Volmer–Heyrovsky pathway, and the Heyrovsky step is a rate-determining step. In this paper, the Pd alloy electrode has exceeded the commercial Pt/C in both acidic and alkaline solutions, which makes it more practical.
中文翻译:

纳米PdFe合金组装膜作为在酸性和碱性溶液中均能高效催化氢生成的电催化剂
在高强度磁场的作用下,通过高真空磁控溅射制备了一系列不同比例的纳米PdFe合金薄膜电极。其中,Pd 3 Fe合金膜电极具有最佳的析氢活性,从而显着改变了Pd的氢吸附(H ad)和吸收(H abs)性质。Pd 3 Fe是一种纳米组装的薄膜,由许多连接在一起的微小纳米片组成。Pd 3 Fe电极的电化学表面积分别是Pd / C和Pd电极的13倍和7倍。同时,Pd和Fe形成了合金结构。Pd 3的(111)平面铁合金是明显的正位移,并且晶胞被压缩。电子通过XPS分析从Fe转移到Pd,证明了Pd和Fe之间的电子关系。DFT计算还显示,与Pd相比,Pd 3 Fe表面的ΔG H *明显降低。降低H在Pd表面上的解吸能有利于提高电催化性能。值得注意的是,在0.5 MH 2 SO 4和1.0 M KOH中,Pd 3 Fe电极的HER活性均超过了Pd / C,Pd和Pt / C。Pd 3的HER活性Fe电极是在-0.2 V的1.0 M KOH中的Pd / C,Pd和Pt / C的20倍,1.9倍和3.1倍。在0.5 MH 2 SO 4中,Pd 3 Fe电极的HER活性是6,是Pd / C,Pd和Pt / C的3.5倍和1.6倍。Pd 3 Fe薄膜电极具有最有利的反应动力学,在酸性和碱性溶液中的Tafel斜率分别为44.7和38.7 mV dec –1。Tafel斜率的值表明,Pd 3 Fe膜电极上HER的机理与Volmer-Heyrovsky途径一致,而Heyrovsky步骤是速率确定步骤。本文中,Pd合金电极在酸性和碱性溶液中都超过了商用Pt / C,这使其更加实用。
更新日期:2020-09-28
中文翻译:

纳米PdFe合金组装膜作为在酸性和碱性溶液中均能高效催化氢生成的电催化剂
在高强度磁场的作用下,通过高真空磁控溅射制备了一系列不同比例的纳米PdFe合金薄膜电极。其中,Pd 3 Fe合金膜电极具有最佳的析氢活性,从而显着改变了Pd的氢吸附(H ad)和吸收(H abs)性质。Pd 3 Fe是一种纳米组装的薄膜,由许多连接在一起的微小纳米片组成。Pd 3 Fe电极的电化学表面积分别是Pd / C和Pd电极的13倍和7倍。同时,Pd和Fe形成了合金结构。Pd 3的(111)平面铁合金是明显的正位移,并且晶胞被压缩。电子通过XPS分析从Fe转移到Pd,证明了Pd和Fe之间的电子关系。DFT计算还显示,与Pd相比,Pd 3 Fe表面的ΔG H *明显降低。降低H在Pd表面上的解吸能有利于提高电催化性能。值得注意的是,在0.5 MH 2 SO 4和1.0 M KOH中,Pd 3 Fe电极的HER活性均超过了Pd / C,Pd和Pt / C。Pd 3的HER活性Fe电极是在-0.2 V的1.0 M KOH中的Pd / C,Pd和Pt / C的20倍,1.9倍和3.1倍。在0.5 MH 2 SO 4中,Pd 3 Fe电极的HER活性是6,是Pd / C,Pd和Pt / C的3.5倍和1.6倍。Pd 3 Fe薄膜电极具有最有利的反应动力学,在酸性和碱性溶液中的Tafel斜率分别为44.7和38.7 mV dec –1。Tafel斜率的值表明,Pd 3 Fe膜电极上HER的机理与Volmer-Heyrovsky途径一致,而Heyrovsky步骤是速率确定步骤。本文中,Pd合金电极在酸性和碱性溶液中都超过了商用Pt / C,这使其更加实用。















































 京公网安备 11010802027423号
京公网安备 11010802027423号