当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isoxazole derivatives of silatrane: synthesis, characterization, in silico ADME profile, prediction of potential pharmacological activity and evaluation of antimicrobial action
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-08-15 , DOI: 10.1002/aoc.5976 Sergey N. Adamovich 1 , Evgeniy V. Kondrashov 1 , Igor A. Ushakov 1 , Nina S. Shatokhina 1 , Elizaveta N. Oborina 1 , Alexander V. Vashchenko 1 , Lydmila A. Belovezhets 1 , Igor B. Rozentsveig 1 , Francis Verpoort 2
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-08-15 , DOI: 10.1002/aoc.5976 Sergey N. Adamovich 1 , Evgeniy V. Kondrashov 1 , Igor A. Ushakov 1 , Nina S. Shatokhina 1 , Elizaveta N. Oborina 1 , Alexander V. Vashchenko 1 , Lydmila A. Belovezhets 1 , Igor B. Rozentsveig 1 , Francis Verpoort 2
Affiliation
|
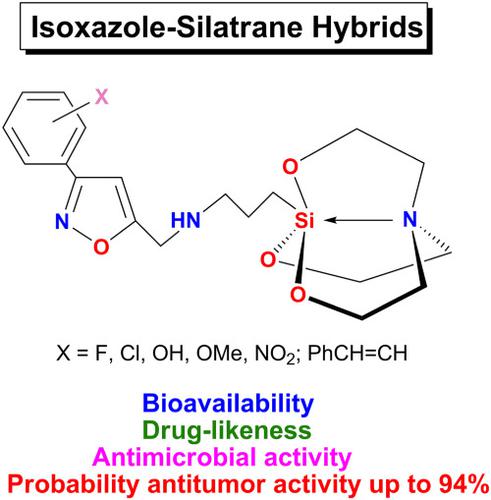
A new family of mono‐ (3a‐h) and bis‐ (4a‐g) isoxazole‐bridged silatranes has been synthesized by the reaction of 3‐aminopropylsilatrane (1) and 3‐substituted 5‐chloro‐methylisoxazoles (2a‐h). The structure of the isoxazole‐silatrane hybrids is characterized by elemental analysis, FT‐IR, UV, NMR (1H, 13C,29Si and 15N) spectroscopy, high‐resolution mass spectrometry, and X‐ray diffraction analysis. The in silico ADME (absorption, distribution, metabolism, excretion) assessment reveals that properties of mono‐adducts (3a‐h) are similar to those of drugs obeyed to the Lipinski's rule. The calculated screening of potential pharmacological activity profiles (in silico PASS program) of isoxazole‐silatranes shows that all synthesized compounds (both mono‐ and bis‐substituted) may have high antitumor action, unlike starting isoxazoles. The preliminary screening of the synthesized silatranes for antimicrobial activity against Enterococcus durans, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa indicates that all test samples are active only against gram‐positive microorganisms. Silatrane 3f displays minimal inhibitory concentration (MIC 12.5 and 6.2 μg ml−1) against E. durans and B. subtilis as compared with standard drug gentamicin (MIC 25 and 50 μg ml−1).
中文翻译:

Silatrane的异恶唑衍生物:合成,表征,计算机模拟ADME分布,潜在药理活性预测和抗菌作用评估
通过3-氨基丙基硅拉(1)与3-取代的5-氯甲基异恶唑(2a-h)的反应合成了一个新的单(3a-h)和双(4a-g)异恶唑桥连的硅杂环戊烷。。异恶唑-硅氮杂杂化物的结构通过元素分析,FT-IR,UV,NMR(1 H,13 C,29 Si和15 N)光谱,高分辨率质谱和X射线衍射分析来表征。在计算机模拟ADME(吸收,分布,代谢,排泄)后发现单加合物(3a-h)类似于服从Lipinski规则的药物。计算得出的对异恶唑-silatranes潜在药理活性谱的筛选(在计算机PASS程序中)显示,与起始的异恶唑不同,所有合成的化合物(单取代和双取代)都可能具有较高的抗肿瘤作用。针对抗微生物活性的合成silatranes的初步筛选肠球菌durans,枯草芽孢杆菌,大肠杆菌,和铜绿假单胞菌表明所有测试样品都是有效只对革兰氏阳性微生物。Silatrane 3f表现出最小的抑制浓度(MIC 12.5和6.2μgml -1)与标准药物庆大霉素(MIC 25和50μgml -1)相比,能抵抗杜兰氏大肠杆菌和枯草芽孢杆菌。
更新日期:2020-08-15
中文翻译:

Silatrane的异恶唑衍生物:合成,表征,计算机模拟ADME分布,潜在药理活性预测和抗菌作用评估
通过3-氨基丙基硅拉(1)与3-取代的5-氯甲基异恶唑(2a-h)的反应合成了一个新的单(3a-h)和双(4a-g)异恶唑桥连的硅杂环戊烷。。异恶唑-硅氮杂杂化物的结构通过元素分析,FT-IR,UV,NMR(1 H,13 C,29 Si和15 N)光谱,高分辨率质谱和X射线衍射分析来表征。在计算机模拟ADME(吸收,分布,代谢,排泄)后发现单加合物(3a-h)类似于服从Lipinski规则的药物。计算得出的对异恶唑-silatranes潜在药理活性谱的筛选(在计算机PASS程序中)显示,与起始的异恶唑不同,所有合成的化合物(单取代和双取代)都可能具有较高的抗肿瘤作用。针对抗微生物活性的合成silatranes的初步筛选肠球菌durans,枯草芽孢杆菌,大肠杆菌,和铜绿假单胞菌表明所有测试样品都是有效只对革兰氏阳性微生物。Silatrane 3f表现出最小的抑制浓度(MIC 12.5和6.2μgml -1)与标准药物庆大霉素(MIC 25和50μgml -1)相比,能抵抗杜兰氏大肠杆菌和枯草芽孢杆菌。















































 京公网安备 11010802027423号
京公网安备 11010802027423号