当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulation of Brønsted acid sites in H‐MOR for selective methyl methoxyacetate synthesis
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-07-09 , DOI: 10.1002/aoc.5925
Jie Yao 1, 2 , Yan Wang 1, 2 , Suleiman Sabo Bello 1, 3 , Guangwen Xu 1, 3 , Lei Shi 1, 3
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-07-09 , DOI: 10.1002/aoc.5925
Jie Yao 1, 2 , Yan Wang 1, 2 , Suleiman Sabo Bello 1, 3 , Guangwen Xu 1, 3 , Lei Shi 1, 3
Affiliation
|
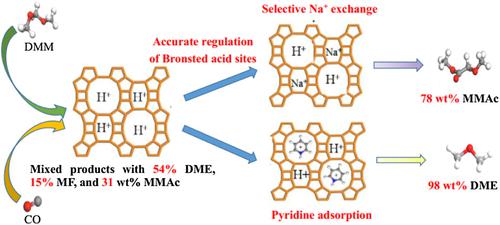
As it is well known, Brønsted acid sites in 8‐MR of H‐MOR (mordenite) are selective for dimethyl ether (DME) carbonylation to methyl acetate, whereas those in 12‐MR are more prone to methanol to olefin reaction. Interestingly, we observed that the Brønsted acid sites in 12‐MR of H‐MOR are highly active for dimethoxymethane (DMM) carbonylation to methyl methoxyacetate (MMAc), whereas those in 8‐MR led to the formation of DME. A series of modified H‐MOR catalysts with accurate regulation of Brønsted acid sites in 12‐MR or 8‐MR were successfully synthesized by selective Na+ exchange or pyridine (Py) adsorption. Fourier‐transform infrared (FT‐IR) spectra, NH3‐temperature‐programmed desorption, Py‐FT‐IR, and inductively coupled plasma analyses suggested that Na+ first occupied Brønsted acid sites in 8‐MR and then replaced those in 12‐MR. All Na+‐exchanged catalysts exhibited significant acceleration on MMAc selectivity, and the ratio of Brønsted acid amount in 12‐MR/total had a positive correlation with MMAc selectivity. The MMAc selectivity (78%) of H‐MOR‐0.15Na was nearly 2.5 times more than that of untreated H‐MOR (31%). However, H‐MOR‐Py showed almost no carbonylation activity (<1% MMAc) and a highest DME selectivity (98%), indicating that Brønsted acid sites in 12‐MR were the only active sites for DMM carbonylation, whereas those in 8‐MR tended to accelerate DMM disproportionation to DME.
中文翻译:

调节H‐MOR中Brønsted酸位以选择性合成甲氧基乙酸甲酯
众所周知,H-MOR(丝光沸石)的8-MR中的布朗斯台德酸位对二甲醚(DME)羰基化为乙酸甲酯具有选择性,而12-MR中的布朗斯台德酸位更易于甲醇转化为烯烃。有趣的是,我们观察到H-MOR的12-MR中的Brønsted酸位对于二甲氧基甲烷(DMM)羰基化为甲氧基乙酸甲酯(MMAc)具有高活性,而8-MR中的布朗斯台德酸位则导致DME的形成。通过选择性的Na +交换或吡啶(Py)吸附,成功合成了一系列能够精确调节12-MR或8-MR中布朗斯台德酸位的改性H-MOR催化剂。傅立叶变换红外(FT-IR)光谱,NH 3程序升温脱附,Py-FT-IR和电感耦合等离子体分析表明,Na +首先在8-MR中占据了布朗斯台德酸性位置,然后取代了12-MR中的酸性位置。所有用Na +交换的催化剂均对MMAc选择性表现出显着的促进作用,并且布朗斯台德酸量的比例在12-MR /总浓度中与MMAc选择性呈正相关。H-MOR-0.15Na的MMAc选择性(78%)是未经处理的H-MOR(31%)的近2.5倍。但是,H‐MOR‐Py几乎没有羰基化活性(<1%MMAc)和最高的DME选择性(98%),表明12‐MR中的布朗斯台德酸位点是DMM羰基化的唯一活性位点,而8‐8 ‐MR倾向于加速DMM向DME的歧化。
更新日期:2020-07-09
中文翻译:

调节H‐MOR中Brønsted酸位以选择性合成甲氧基乙酸甲酯
众所周知,H-MOR(丝光沸石)的8-MR中的布朗斯台德酸位对二甲醚(DME)羰基化为乙酸甲酯具有选择性,而12-MR中的布朗斯台德酸位更易于甲醇转化为烯烃。有趣的是,我们观察到H-MOR的12-MR中的Brønsted酸位对于二甲氧基甲烷(DMM)羰基化为甲氧基乙酸甲酯(MMAc)具有高活性,而8-MR中的布朗斯台德酸位则导致DME的形成。通过选择性的Na +交换或吡啶(Py)吸附,成功合成了一系列能够精确调节12-MR或8-MR中布朗斯台德酸位的改性H-MOR催化剂。傅立叶变换红外(FT-IR)光谱,NH 3程序升温脱附,Py-FT-IR和电感耦合等离子体分析表明,Na +首先在8-MR中占据了布朗斯台德酸性位置,然后取代了12-MR中的酸性位置。所有用Na +交换的催化剂均对MMAc选择性表现出显着的促进作用,并且布朗斯台德酸量的比例在12-MR /总浓度中与MMAc选择性呈正相关。H-MOR-0.15Na的MMAc选择性(78%)是未经处理的H-MOR(31%)的近2.5倍。但是,H‐MOR‐Py几乎没有羰基化活性(<1%MMAc)和最高的DME选择性(98%),表明12‐MR中的布朗斯台德酸位点是DMM羰基化的唯一活性位点,而8‐8 ‐MR倾向于加速DMM向DME的歧化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号