当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complexes of HArF and AuX (X = F, Cl, Br, I). Comparison of H‐bonds, halogen bonds, F‐shared bonds and covalent bonds
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-06-22 , DOI: 10.1002/aoc.5891 Ruijing Wang 1 , Qingzhong Li 1 , Steve Scheiner 2
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-06-22 , DOI: 10.1002/aoc.5891 Ruijing Wang 1 , Qingzhong Li 1 , Steve Scheiner 2
Affiliation
|
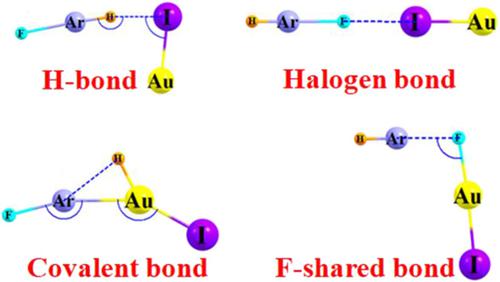
The various sorts of complexes in which HArF and AuX (X = F, Cl, Br, I) can engage are probed by MP2/aug‐cc‐pVTZ calculations. The most weakly bound are those containing a halogen bond (XB) of the AuX⋯FArH sort, with binding energies less than 8 kcal/mol. H‐bonded dimers FArH⋯XAu are a little stronger, held together by some 12 kcal/mol. Being the most strongly bound places the F atom of HArF roughly midway between Ar and Au in an F‐shaped structure, bound by some 43–54 kcal/mol. The last sort of product involves atomic rearrangements wherein the H atom migrates from Ar to Au, followed by formation of a covalent Ar–Au bond. The resulting molecular unit is stabilized by 30–40 kcal/mol relative to the original HArF and AuX reactants. The H‐bonded dimers are held together by an unusually large polarization component, surpassing electrostatic attraction, while dispersion predominates for the halogen bonds. Perturbations of the geometries and stretching frequencies offer a ready means of distinguishing the different types of complexes by spectroscopic techniques.
中文翻译:

HArF和AuX的配合物(X = F,Cl,Br,I)。H键,卤素键,F共享键和共价键的比较
MP2 / aug-cc-pVTZ计算可探查HArF和AuX(X = F,Cl,Br,I)可以结合的各种复合物。结合最弱的是含有AuX⋯FArH类卤素键(XB)的那些,其结合能小于8 kcal / mol。H键合二聚体FArH⋯XAu稍强一点,约12 kcal / mol。作为最牢固的键合,HArF的F原子大约处于Ar和Au之间的F形结构的中间,键合约43–54 kcal / mol。最后一种产物涉及原子重排,其中H原子从Ar迁移到Au,然后形成共价Ar-Au键。相对于最初的HArF和AuX反应物,所得分子单元稳定在30–40 kcal / mol。H键合的二聚体通过异常大的偏振分量结合在一起,超过静电吸引,而卤素键主要是分散。几何形状和拉伸频率的扰动提供了一种通过光谱技术区分不同类型复合物的简便方法。
更新日期:2020-06-22
中文翻译:

HArF和AuX的配合物(X = F,Cl,Br,I)。H键,卤素键,F共享键和共价键的比较
MP2 / aug-cc-pVTZ计算可探查HArF和AuX(X = F,Cl,Br,I)可以结合的各种复合物。结合最弱的是含有AuX⋯FArH类卤素键(XB)的那些,其结合能小于8 kcal / mol。H键合二聚体FArH⋯XAu稍强一点,约12 kcal / mol。作为最牢固的键合,HArF的F原子大约处于Ar和Au之间的F形结构的中间,键合约43–54 kcal / mol。最后一种产物涉及原子重排,其中H原子从Ar迁移到Au,然后形成共价Ar-Au键。相对于最初的HArF和AuX反应物,所得分子单元稳定在30–40 kcal / mol。H键合的二聚体通过异常大的偏振分量结合在一起,超过静电吸引,而卤素键主要是分散。几何形状和拉伸频率的扰动提供了一种通过光谱技术区分不同类型复合物的简便方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号