Cellular and Molecular Gastroenterology and Hepatology ( IF 7.1 ) Pub Date : 2020-08-19 , DOI: 10.1016/j.jcmgh.2020.08.003 Rui Sun 1 , Matija Hedl 1 , Clara Abraham 1
|
Background & Aims
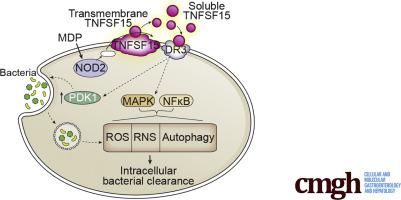
TNFSF15 genetic variants leading to increased TNF superfamily member 15 (TNFSF15) expression confer risk for inflammatory bowel disease (IBD), and TNFSF15 is being explored as a therapeutic target in IBD patients. Although the focus for TNFSF15-mediated inflammatory outcomes has been predominantly on its action on T cells, TNFSF15 also promotes inflammatory outcomes in human macrophages. Given the critical role for macrophages in bacterial clearance, we hypothesized that TNFSF15 promotes antimicrobial pathways in human macrophages and that macrophages from TNFSF15 IBD risk carriers with higher TNFSF15 expression have an advantage in these antimicrobial outcomes.
Methods
We analyzed protein expression, signaling, bacterial uptake, and intracellular bacterial clearance in human monocyte-derived macrophages through flow cytometry, enzyme-linked immunosorbent assay, and gentamicin protection.
Results
Autocrine/paracrine TNFSF15 interactions with death receptor 3 (DR3) were required for optimal levels of pattern-recognition-receptor (PRR)-induced bacterial clearance in human macrophages. TNFSF15 induced pyruvate dehydrogenase kinase 1-dependent bacterial uptake and promoted intracellular bacterial clearance through reactive oxygen species, nitric oxide synthase 2, and autophagy up-regulation. The TNFSF15-initiated TNF receptor-associated factor 2/receptor-interacting protein kinase 1/RIP3 pathway was required for mitogen-activated protein kinase and nuclear factor-κB activation, and, in turn, induction of each of the antimicrobial pathways; the TNFSF15-initiated Fas-associated protein with death domain/mucosa-associated lymphoid tissue lymphoma translocation protein 1/caspase-8 pathway played a less prominent role in antimicrobial functions, despite its key role in TNFSF15-induced cytokine secretion. Complementation of signaling pathways or antimicrobial pathways restored bacterial uptake and clearance in PRR-stimulated macrophages where TNFSF15:DR3 interactions were inhibited. Monocyte-derived macrophages from high TNFSF15-expressing rs6478108 TT IBD risk carriers in the TNFSF15 region showed increased levels of the identified antimicrobial pathways.
Conclusions
We identify that autocrine/paracrine TNFSF15 is required for optimal PRR-enhanced antimicrobial pathways in macrophages, define mechanisms regulating TNFSF15-dependent bacterial clearance, and determine how the TNFSF15 IBD risk genotype modulates these outcomes.
中文翻译:

TNFSF15 促进人类巨噬细胞中的抗菌通路,这些通路受 TNFSF15 疾病风险变体的调节。
背景与目标
导致 TNF 超家族成员 15 (TNFSF15) 表达增加的TNFSF15遗传变异赋予炎症性肠病 (IBD) 风险,并且正在探索将 TNFSF15 作为 IBD 患者的治疗靶点。尽管 TNFSF15 介导的炎症结果的重点主要在于其对 T 细胞的作用,但 TNFSF15 也促进人类巨噬细胞的炎症结果。鉴于巨噬细胞在细菌清除中的关键作用,我们假设 TNFSF15 促进人类巨噬细胞中的抗菌途径,并且来自具有较高 TNFSF15 表达的TNFSF15 IBD 风险携带者的巨噬细胞在这些抗菌结果中具有优势。
方法
我们通过流式细胞术、酶联免疫吸附测定和庆大霉素保护分析了人单核细胞衍生的巨噬细胞中的蛋白质表达、信号传导、细菌摄取和细胞内细菌清除。
结果
自分泌/旁分泌 TNFSF15 与死亡受体 3 (DR3) 的相互作用是人类巨噬细胞中模式识别受体 (PRR) 诱导的细菌清除的最佳水平所必需的。TNFSF15 诱导丙酮酸脱氢酶激酶 1 依赖性细菌摄取,并通过活性氧、一氧化氮合酶 2 和自噬上调促进细胞内细菌清除。TNFSF15 启动的 TNF 受体相关因子 2/受体相互作用蛋白激酶 1/RIP3 途径是丝裂原活化蛋白激酶和核因子-κB 激活所必需的,进而诱导每种抗菌途径;TNFSF15 启动的 Fas 相关蛋白与死亡域/粘膜相关淋巴组织淋巴瘤易位蛋白 1/caspase-8 通路在抗菌功能中的作用不太突出,尽管它在 TNFSF15 诱导的细胞因子分泌中起关键作用。信号通路或抗菌通路的补充恢复了 PRR 刺激的巨噬细胞中的细菌摄取和清除,其中 TNFSF15:DR3 相互作用被抑制。来自高表达 TNFSF15 rs6478108 TT IBD 风险携带者的单核细胞衍生巨噬细胞TNFSF15区域显示出已鉴定的抗菌途径的水平增加。
结论
我们确定自分泌/旁分泌 TNFSF15 是巨噬细胞中最佳 PRR 增强抗菌途径所必需的,定义了调节 TNFSF15 依赖性细菌清除的机制,并确定了TNFSF15 IBD 风险基因型如何调节这些结果。































 京公网安备 11010802027423号
京公网安备 11010802027423号