Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-08-20 , DOI: 10.1016/j.tetlet.2020.152366 Wenjing Bai , Jianxin Ji , Qiang Huang , Wei Wei
|
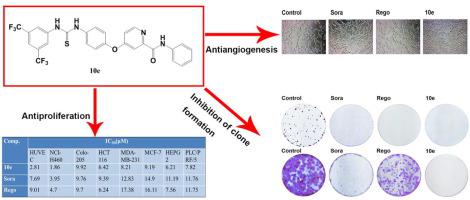
A series of novel thiourea derivatives were synthesized and evaluated by biological activities. Among them, compound 10e containing 3,5-bis(trifluoromethyl)phenyl moiety (R1) at the terminal thiourea and phenylamino (R2) at the terminal acyl position showed the best cytotoxic activities against seven cancer cell lines (NCI-H460, Colo-205, HCT116, MDA-MB-231, MCF-7, HepG2, PLC/PRF/5) and HUVECs. Moreover, compound 10e moderately inhibited various RTKs such as VEGFR2, VEGFR3, and PDGFRβ. Notably, 10e exhibited much better inhibitory effect of tumor formation and antiangiogenic activities than Sorafenib and Regorafenib at the same concentration. Further docking studies suggested that 10e could serve as potential candidate for cancer therapy.
中文翻译:

新型硫脲衍生物作为抗肿瘤和抗血管生成剂的合成与评价
合成了一系列新型硫脲衍生物,并通过生物学活性对其进行了评估。其中,在末端硫脲处含有3,5-双(三氟甲基)苯基部分(R 1)在酰基末端具有苯氨基(R 2)的化合物10e对7种癌细胞系(NCI-H460, Colo-205,HCT116,MDA-MB-231,MCF-7,HepG2,PLC / PRF / 5)和HUVEC。此外,化合物10e适度抑制各种RTK,例如VEGFR2,VEGFR3和PDGFRβ。值得注意的是,在相同浓度下,10e对肿瘤形成和抗血管生成活性的抑制作用比索拉非尼和Regorafenib好得多。进一步的对接研究表明10e 可以作为癌症治疗的潜在候选人。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号