当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overall Structures of Two Metal Nanoclusters: Chloride as a Bridge Fills the Space between the Metal Core and the Metal Shell.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-08-18 , DOI: 10.1021/acs.inorgchem.0c01638 Xuejuan Zou 1, 2 , Shan Jin 2, 3 , Xiao Wei 1, 2 , Xiaowu Li 1, 2 , Manman Zhou 1, 2 , Shuxin Wang 1, 2 , Manzhou Zhu 1, 2, 3
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-08-18 , DOI: 10.1021/acs.inorgchem.0c01638 Xuejuan Zou 1, 2 , Shan Jin 2, 3 , Xiao Wei 1, 2 , Xiaowu Li 1, 2 , Manman Zhou 1, 2 , Shuxin Wang 1, 2 , Manzhou Zhu 1, 2, 3
Affiliation
|
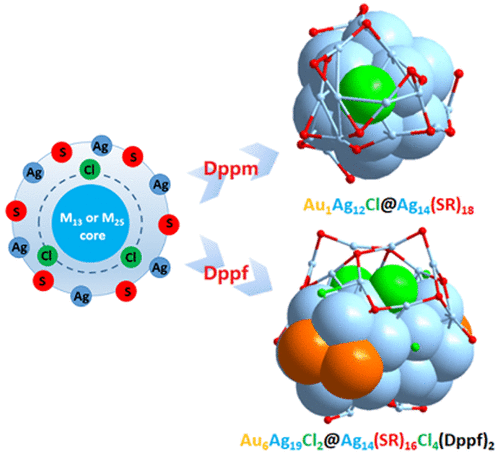
In addition to using common ligands (phosphine, thiol, or acetylene ligands) to protect metal nanoclusters, halogens can also be used to participate in the formation of nanoclusters. In this study, we reported the formation of two new nanoclusters promoted by chlorides released from HAuCl4: one [Au1Ag26(SR)18Cl] with an icosahedral Au1Ag12 kernel, which is surrounded by the shell of Ag14(SR)18, and the special chlorine atom fills the space between the metal core and the metal shell; the other [Au6Ag33(Dppf)2(SR)16Cl6]+ with the kernel consisted of two icosahedral Au3Ag10 units by sharing one vertex Ag atom, which is protected by the complicated shell of Ag14(Dppf)2(SR)16Cl4, two special chlorine atoms also fill the space between the metal core and the metal shell. Thus, both two nanoclusters suggest that the chlorine atoms can exist in the space between the metal kernel and out shell, playing a critical role in maintaining the stability of the overall structures. These will deepen the comprehensive understanding of chlorine in constructing the structures of alloy nanoclusters and will also be helpful in mapping out the new strategies for core–shell nanocluster synthesis.
中文翻译:

两个金属纳米簇的整体结构:氯化物作为桥填充金属核和金属壳之间的空间。
除了使用常见的配体(膦,硫醇或乙炔配体)保护金属纳米团簇之外,卤素还可以用于参与纳米团簇的形成。在这项研究中,我们报道了由HAuCl 4释放的氯化物促进形成的两个新的纳米团簇:一个[Au 1 Ag 26(SR)18 Cl],具有二十面体的Au 1 Ag 12核,被Ag 14的壳包围(SR)18,特殊的氯原子填充金属芯和金属壳之间的空间;另一个[Au 6 Ag 33(Dppf)2(SR)16 Cl 6] +的内核由两个二十面体Au 3 Ag 10单元组成,它们共享一个顶点Ag原子,该原子受到复杂的Ag 14(Dppf)2(SR)16 Cl 4壳的保护。,两个特殊的氯原子也填充了金属核和金属壳之间的空间。因此,两个纳米团簇都表明氯原子可以存在于金属核与外壳之间的空间中,在维持整体结构的稳定性方面起着至关重要的作用。这些将加深对氯在构造合金纳米团簇结构中的全面理解,还将有助于为核-壳纳米团簇的合成制定新的策略。
更新日期:2020-09-08
中文翻译:

两个金属纳米簇的整体结构:氯化物作为桥填充金属核和金属壳之间的空间。
除了使用常见的配体(膦,硫醇或乙炔配体)保护金属纳米团簇之外,卤素还可以用于参与纳米团簇的形成。在这项研究中,我们报道了由HAuCl 4释放的氯化物促进形成的两个新的纳米团簇:一个[Au 1 Ag 26(SR)18 Cl],具有二十面体的Au 1 Ag 12核,被Ag 14的壳包围(SR)18,特殊的氯原子填充金属芯和金属壳之间的空间;另一个[Au 6 Ag 33(Dppf)2(SR)16 Cl 6] +的内核由两个二十面体Au 3 Ag 10单元组成,它们共享一个顶点Ag原子,该原子受到复杂的Ag 14(Dppf)2(SR)16 Cl 4壳的保护。,两个特殊的氯原子也填充了金属核和金属壳之间的空间。因此,两个纳米团簇都表明氯原子可以存在于金属核与外壳之间的空间中,在维持整体结构的稳定性方面起着至关重要的作用。这些将加深对氯在构造合金纳米团簇结构中的全面理解,还将有助于为核-壳纳米团簇的合成制定新的策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号