当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidation of Fe(II) by Flavins under Anoxic Conditions.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-08-19 , DOI: 10.1021/acs.est.0c02916
Peng Zhang 1 , Philippe Van Cappellen 2 , Kunfu Pi 2 , Songhu Yuan 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-08-19 , DOI: 10.1021/acs.est.0c02916
Peng Zhang 1 , Philippe Van Cappellen 2 , Kunfu Pi 2 , Songhu Yuan 1
Affiliation
|
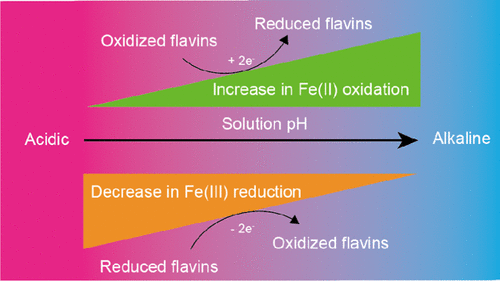
Flavin-mediated electron transfer is an important pathway for Fe(III) reduction by dissimilatory iron-reducing bacteria. Although the mechanisms and kinetics of Fe(III) reduction by reduced flavins have been widely studied, the reaction between Fe(II) and oxidized flavins is rarely investigated. Results of this study show that under anoxic conditions, Fe(II) can be oxidized by the oxidized forms of riboflavin (RBF) and flavin mononucleotide (FMN) at pH 7–9. For instance, at pH 9, 73% of 17.8 μM Fe(II) was oxidized by 10 μM RBF within 20 min. Both the rate and extent of oxidation increased with increasing concentrations of oxidized flavins and increasing solution pH. Thermodynamic calculations and kinetic analyses implied that the oxidation of Fe(II) proceeded predominantly via the autodecomposition of Fe2+–RBF– and Fe2+–FMN– complexes, along with minor contributions from direct oxidation of Fe(II) by flavins and flavin radicals. Our findings suggest that the reoxidation of Fe(II) by oxidized flavins may be a rate-controlling factor in microbial Fe(III) reduction via flavin-mediated electron transfer.
中文翻译:

黄素在缺氧条件下氧化Fe(II)。
黄素介导的电子转移是异化铁还原细菌还原Fe(III)的重要途径。尽管已广泛研究了还原黄酮还原Fe(III)的机理和动力学,但很少研究Fe(II)与氧化黄素之间的反应。这项研究的结果表明,在缺氧条件下,Fe(II)可以在pH 7-9下被核黄素(RBF)和黄素单核苷酸(FMN)的氧化形式氧化。例如,在pH 9下,在20分钟内73%的17.8μMFe(II)被10μMRBF氧化。氧化速率和程度均随氧化黄素浓度的增加和溶液pH值的增加而增加。热力学计算和动力学分析表明Fe(II)的氧化主要通过Fe 2+ –RBF的自分解进行-和Fe 2+ -FMN -复合物,由黄素和黄素自由基从铁(II)的直接氧化轻微的贡献一起。我们的发现表明,氧化黄素对Fe(II)的再氧化可能是微生物通过黄素介导的电子转移还原Fe(III)的速率控制因素。
更新日期:2020-09-15
中文翻译:

黄素在缺氧条件下氧化Fe(II)。
黄素介导的电子转移是异化铁还原细菌还原Fe(III)的重要途径。尽管已广泛研究了还原黄酮还原Fe(III)的机理和动力学,但很少研究Fe(II)与氧化黄素之间的反应。这项研究的结果表明,在缺氧条件下,Fe(II)可以在pH 7-9下被核黄素(RBF)和黄素单核苷酸(FMN)的氧化形式氧化。例如,在pH 9下,在20分钟内73%的17.8μMFe(II)被10μMRBF氧化。氧化速率和程度均随氧化黄素浓度的增加和溶液pH值的增加而增加。热力学计算和动力学分析表明Fe(II)的氧化主要通过Fe 2+ –RBF的自分解进行-和Fe 2+ -FMN -复合物,由黄素和黄素自由基从铁(II)的直接氧化轻微的贡献一起。我们的发现表明,氧化黄素对Fe(II)的再氧化可能是微生物通过黄素介导的电子转移还原Fe(III)的速率控制因素。




































 京公网安备 11010802027423号
京公网安备 11010802027423号