当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phenyl-Bridged Graphitic Carbon Nitride with a Porous and Hollow Sphere Structure to Enhance Dissociation of Photogenerated Charge Carriers and Visible-Light-Driven H2 Generation.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-08-19 , DOI: 10.1021/acsami.0c11578 Yanglin Chen 1 , Ye Qu 1 , Xin Zhou 1 , Dazhi Li 1 , Ping Xu 1 , Jianmin Sun 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-08-19 , DOI: 10.1021/acsami.0c11578 Yanglin Chen 1 , Ye Qu 1 , Xin Zhou 1 , Dazhi Li 1 , Ping Xu 1 , Jianmin Sun 1
Affiliation
|
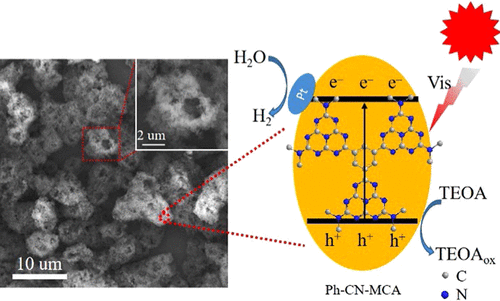
Graphitic carbon nitride (CN) suffers from rapid recombination of photoexcited charges due to the existing highly symmetrical tri-s-triazine ring and long charge diffusion path, resulting in moderate photocatalytic activity. The bridged phenyl embedded in the CN structure was used to reduce the symmetry of the tri-s-triazine ring. In addition, the CN material was constructed with a porous and hollow sphere structure to shorten the diffusion path of charge carriers. Herein, simple thermal polymerization of a trimesic acid-doped melamine–cyanuric acid (MCA) supramolecular was employed to construct phenyl-bridged graphitic carbon nitride (Ph-CN-MCA) with a hollow sphere structure composed of porous nanosheets for visible-light catalytic H2 evolution. The porous and hollow sphere-structured Ph-CN-MCA possessed increased degree of polymerization, more negative conduction band potential, enlarged Brunauer–Emmett–Teller (BET) surface area, and shortened charge diffusion path. In addition, bridged phenyl embedded in the Ph-CN-MCA structure not only accelerated the dissociation of photogenerated carriers but also narrowed the band gap and extended the visible-light absorption. Further, the separated highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of Ph-CN-MCA facilitated the spatial dissociation of photogenerated charges, which was also confirmed by theoretical calculations. As a consequence, compared with the reference CN-MA catalyst prepared from melamine, Ph-CN-MCA showed approximately 48.42 times the photocatalytic H2 evolution under visible-light irradiation. The developed synthetic method herein highlights that phenyl-bridged graphitic carbon nitride with a porous and hollow sphere structure could provide an efficient platform to boost the dissociation of photoexcited charge carriers and photocatalytic H2 evolution.
中文翻译:

具有多孔和空心球结构的苯桥型石墨氮化碳,可增强光生载流子的离解和可见光驱动的H2生成。
由于存在高度对称的三-s-三嗪环和较长的电荷扩散路径,石墨化的氮化碳(CN)遭受光激发电荷的快速重组,导致中等的光催化活性。嵌入CN结构中的桥联苯基用于降低三-s-三嗪环的对称性。另外,CN材料被构造成具有多孔和中空的球形结构,以缩短电荷载流子的扩散路径。本文中,使用偏苯三酸掺杂的三聚氰胺-氰尿酸(MCA)超分子的简单热聚合反应来构建由多孔纳米片组成的中空球形结构的苯基桥接石墨氮化碳(Ph-CN-MCA),用于可见光催化高2演化。多孔空心球状的Ph-CN-MCA具有更高的聚合度,更大的负导带电势,更大的Brunauer-Emmett-Teller(BET)表面积以及缩短的电荷扩散路径。此外,Ph-CN-MCA结构中嵌入的桥联苯基不仅加速了光生载流子的离解,而且还缩小了带隙并扩展了可见光吸收。此外,Ph-CN-MCA的分离的最高占据分子轨道(HOMO)和最低未占据分子轨道(LUMO)促进了光生电荷的空间解离,这也得到了理论计算的证实。结果,与由三聚氰胺制得的参比CN-MA催化剂相比,Ph-CN-MCA表现出光催化H的约48.42倍2可见光照射下的演变。本文开发的合成方法强调了具有多孔和空心球结构的苯基桥碳氮化碳可以提供一个有效的平台来促进光激发电荷载体的离解和光催化H 2的释放。
更新日期:2020-09-16
中文翻译:

具有多孔和空心球结构的苯桥型石墨氮化碳,可增强光生载流子的离解和可见光驱动的H2生成。
由于存在高度对称的三-s-三嗪环和较长的电荷扩散路径,石墨化的氮化碳(CN)遭受光激发电荷的快速重组,导致中等的光催化活性。嵌入CN结构中的桥联苯基用于降低三-s-三嗪环的对称性。另外,CN材料被构造成具有多孔和中空的球形结构,以缩短电荷载流子的扩散路径。本文中,使用偏苯三酸掺杂的三聚氰胺-氰尿酸(MCA)超分子的简单热聚合反应来构建由多孔纳米片组成的中空球形结构的苯基桥接石墨氮化碳(Ph-CN-MCA),用于可见光催化高2演化。多孔空心球状的Ph-CN-MCA具有更高的聚合度,更大的负导带电势,更大的Brunauer-Emmett-Teller(BET)表面积以及缩短的电荷扩散路径。此外,Ph-CN-MCA结构中嵌入的桥联苯基不仅加速了光生载流子的离解,而且还缩小了带隙并扩展了可见光吸收。此外,Ph-CN-MCA的分离的最高占据分子轨道(HOMO)和最低未占据分子轨道(LUMO)促进了光生电荷的空间解离,这也得到了理论计算的证实。结果,与由三聚氰胺制得的参比CN-MA催化剂相比,Ph-CN-MCA表现出光催化H的约48.42倍2可见光照射下的演变。本文开发的合成方法强调了具有多孔和空心球结构的苯基桥碳氮化碳可以提供一个有效的平台来促进光激发电荷载体的离解和光催化H 2的释放。






























 京公网安备 11010802027423号
京公网安备 11010802027423号