当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Measurement and Correlation of CO2 in bis(pentafluoroethylsulfonyl)imide ([BETI]) Anion-Based Ionic Liquids: [EMIM][BETI], [BMIM][BETI], [HMIM][BETI]
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-08-18 , DOI: 10.1021/acs.jced.0c00188 Joon-Hyuk Yim 1 , Byung-Keun Oh 1 , Jong Sung Lim 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-08-18 , DOI: 10.1021/acs.jced.0c00188 Joon-Hyuk Yim 1 , Byung-Keun Oh 1 , Jong Sung Lim 1
Affiliation
|
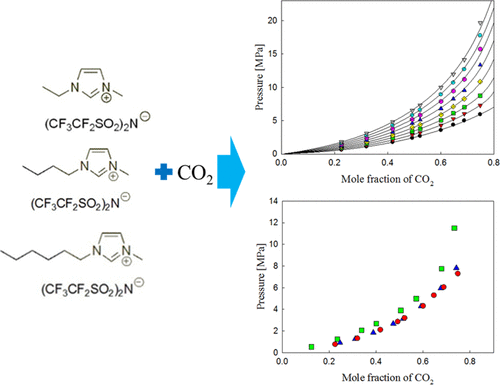
The ionic liquid (IL) + CO2 system was studied for solubility measurement in three distinct [BETI] anion-based ILs: 1-ethyl-3-methylimidazolium bis(pentafluoroethylsulfonyl)imide ([EMIM][BETI]), 1-butyl-3-methylimidazolium bis(pentafluoroethylsulfonyl)imide ([BMIM][BETI]), and 1-hexyl-3-methylimidazolium bis(pentafluoroethylsulfonyl)imide ([HMIM][BETI]) in the experimental ranges of 0.1–29.3 MPa and 303.2–373.2 K. The present study shows the solubility of CO2 in several [BETI] anion-based ILs under specific experimental conditions and compares the effect of three cations: [HMIM], [BMIM], and [EMIM]. CO2 solubility data were measured by determining bubble (or cloud)-point pressures at targeted CO2 mole fractions. It was found that CO2 absorption abilities were placed in the following order: [HMIM][BETI] > [BMIM][BETI] > [EMIM][BETI]. Moreover, these three [BETI]-containing ILs were compared with previously published data for investigating the influence of the [BETI] anion. The result shows that the three [BETI] anion-based ILs have relatively higher solubility for CO2 than any other ILs discussed in this paper. These experimental results also show that the larger amount of the fluoro-alkyl group containing anion increases CO2 solubility. To correlate and calculate the resulting data, the following methods were used: the PR-EoS with one-fluid mixing rule and the modified Lydersen–Joback–Reid method. The overall absolute average relative deviation were 0.0172, 0.0246, and 0.0118 for CO2 + [EMIM][BETI], CO2 + [BMIM][BETI], and CO2 + [HMIM][BETI] systems, respectively.
中文翻译:

CO 2在双(五氟乙基磺酰基)亚胺([BETI])阴离子型离子液体中的溶解度测量和相关性:[EMIM] [BETI],[BMIM] [BETI],[HMIM] [BETI]
研究了离子液体(IL)+ CO 2系统在三种基于[BETI]阴离子的不同IL中的溶解度测量:1-乙基-3-甲基咪唑鎓双(五氟乙基磺酰基)酰亚胺([EMIM] [BETI]),1-丁基在0.1–29.3 MPa和303.2的试验范围内,-3-甲基咪唑双(五氟乙基磺酰基)酰亚胺([BMIM] [BETI])和1-己基-3-甲基咪唑双(五氟乙基磺酰基)酰亚胺([HMIM] [BETI])在0.1–29.3 MPa和303.2的实验范围内–373.2K。本研究显示了在特定实验条件下,CO 2在几种基于[BETI]阴离子的ILs中的溶解度,并比较了三种阳离子[HMIM],[BMIM]和[EMIM]的作用。通过确定目标CO 2摩尔分数下的气泡(或浊点)压力来测量CO 2溶解度数据。发现一氧化碳2种吸收能力按以下顺序排列:[HMIM] [BETI]> [BMIM] [BETI]> [EMIM] [BETI]。此外,将这三种含[BETI]的IL与先前发表的数据进行了比较,以研究[BETI]阴离子的影响。结果表明,三种基于[BETI]阴离子的ILs对CO 2的溶解度均比本文讨论的任何其他ILs高。这些实验结果还表明,较大量的含氟烷基的阴离子增加了CO 2的溶解度。为了关联和计算结果数据,使用了以下方法:具有单流体混合规则的PR-EoS和改进的Lydersen-Joback-Reid方法。CO 2的总体绝对平均相对偏差为0.0172、0.0246和0.0118+ [EMIM] [BETI],CO 2 + [BMIM] [BETI]和CO 2 + [HMIM] [BETI]系统。
更新日期:2020-09-10
中文翻译:

CO 2在双(五氟乙基磺酰基)亚胺([BETI])阴离子型离子液体中的溶解度测量和相关性:[EMIM] [BETI],[BMIM] [BETI],[HMIM] [BETI]
研究了离子液体(IL)+ CO 2系统在三种基于[BETI]阴离子的不同IL中的溶解度测量:1-乙基-3-甲基咪唑鎓双(五氟乙基磺酰基)酰亚胺([EMIM] [BETI]),1-丁基在0.1–29.3 MPa和303.2的试验范围内,-3-甲基咪唑双(五氟乙基磺酰基)酰亚胺([BMIM] [BETI])和1-己基-3-甲基咪唑双(五氟乙基磺酰基)酰亚胺([HMIM] [BETI])在0.1–29.3 MPa和303.2的实验范围内–373.2K。本研究显示了在特定实验条件下,CO 2在几种基于[BETI]阴离子的ILs中的溶解度,并比较了三种阳离子[HMIM],[BMIM]和[EMIM]的作用。通过确定目标CO 2摩尔分数下的气泡(或浊点)压力来测量CO 2溶解度数据。发现一氧化碳2种吸收能力按以下顺序排列:[HMIM] [BETI]> [BMIM] [BETI]> [EMIM] [BETI]。此外,将这三种含[BETI]的IL与先前发表的数据进行了比较,以研究[BETI]阴离子的影响。结果表明,三种基于[BETI]阴离子的ILs对CO 2的溶解度均比本文讨论的任何其他ILs高。这些实验结果还表明,较大量的含氟烷基的阴离子增加了CO 2的溶解度。为了关联和计算结果数据,使用了以下方法:具有单流体混合规则的PR-EoS和改进的Lydersen-Joback-Reid方法。CO 2的总体绝对平均相对偏差为0.0172、0.0246和0.0118+ [EMIM] [BETI],CO 2 + [BMIM] [BETI]和CO 2 + [HMIM] [BETI]系统。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号