Cell Reports ( IF 7.5 ) Pub Date : 2020-08-18 , DOI: 10.1016/j.celrep.2020.108042 Frauke Beilstein 1 , Abbas Abou Hamdan 1 , Hélène Raux 1 , Laura Belot 1 , Malika Ouldali 1 , Aurélie A Albertini 1 , Yves Gaudin 1
|
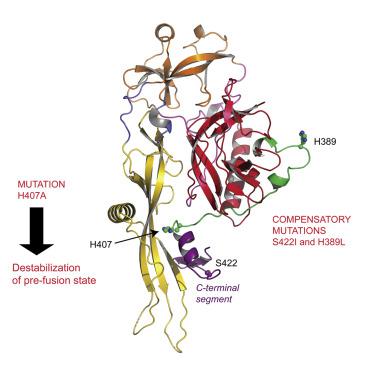
VSV fusion machinery, like that of many other enveloped viruses, is triggered at low pH in endosomes after virion endocytosis. It was suggested that some histidines could play the role of pH-sensitive switches. By mutating histidine residues H22, H60, H132, H162, H389, H397, H407, and H409, we demonstrate that residues H389 and D280, facing each other in the six-helix bundle of the post-fusion state, and more prominently H407, located at the interface between the C-terminal part of the ectodomain and the fusion domain, are crucial for fusion. Passages of recombinant viruses bearing mutant G resulted in the selection of compensatory mutations. Thus, the H407A mutation in G resulted in two independent compensatory mutants, L396I and S422I. Together with a crystal structure of G, presented here, which extends our knowledge of G pre-fusion structure, this indicates that the conformational transition is initiated by refolding of the C-terminal part of the G ectodomain.
中文翻译:

鉴定VSV-G中的pH敏感开关和G融合前状态的晶体结构突出了VSV-G结构的转变途径。
像许多其他包膜病毒的VSV融合机制一样,病毒体内吞后,内体的pH较低时也会触发。有人提出,一些组氨酸可能起pH敏感开关的作用。通过突变组氨酸残基H22,H60,H132,H162,H389,H397,H407和H409,我们证明了残基H389和D280在融合后状态的六螺旋束中相互面对,更重要的是H407,位于胞外域的C-末端部分与融合域之间的界面处的融合对于融合至关重要。携带突变体G的重组病毒的传代导致补偿性突变的选择。因此,G中的H407A突变产生两个独立的补偿性突变体L396I和S422I。与此处介绍的G的晶体结构一起,扩展了我们对G预融合结构的了解,































 京公网安备 11010802027423号
京公网安备 11010802027423号