当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis of Specificity for Carboxyl-terminated Acyl Donors in A Bacterial Acyltransferase
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-17 , DOI: 10.1021/jacs.0c07331 Fei Xiao 1 , Sheng Dong , Yang Liu 1 , Yingang Feng , Huayue Li 1, 2 , Cai-Hong Yun 3 , Qiu Cui , Wenli Li 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-17 , DOI: 10.1021/jacs.0c07331 Fei Xiao 1 , Sheng Dong , Yang Liu 1 , Yingang Feng , Huayue Li 1, 2 , Cai-Hong Yun 3 , Qiu Cui , Wenli Li 1, 2
Affiliation
|
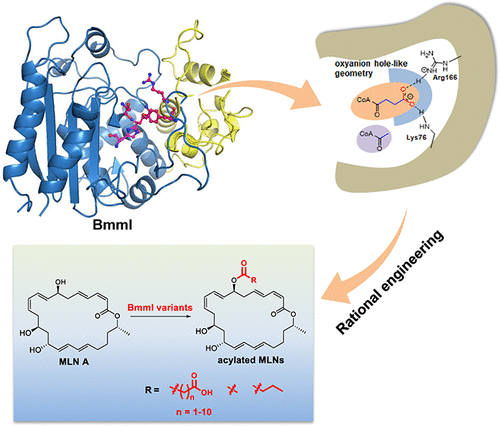
Macrolactins (MLNs) are a class of important antimacular degeneration and antitumor agents. Malonylated/succinylated MLNs are even more important due to their efficacy to overcome multidrug resistant bacteria. However, which enzyme catalyzes this reaction remains enigmatic. Herein, we deciphered a beta-lactamase homolog BmmI to be responsible for this step. BmmI could specifically attach C3-C5 alkyl acid thioesters onto 7-OH of MLN A, and also exhibits substrate promiscuity towards acyl acceptors with different scaffolds. Crystal structure of BmmI covalently linked with succinyl group and systematic mutagenesis highlighted the role of an oxyanion hole-like geometry in the recognition of carboxyl-terminated acyl donors. Engineering of this geometry expanded its substrate scope, with the R166A/G/Q variants recognizing up to C12 alkyl acid thioester. Structure of BmmI with the acyl acceptor MLN A revealed the importance of Arg292 in recognition of the macrolide substrates. Moreover, the mechanism of BmmI-catalyzed acyltransfer reaction was established, unmasking the deft role of Lys76 in governing acyl donors as well as catalysis. Our studies uncover the delicate mechanism underlying substrate selectivity of acyltransferases, which would guide rational enzyme engineering for drug development.
中文翻译:

细菌酰基转移酶中羧基末端酰基供体特异性的结构基础
Macrolactins (MLNs) 是一类重要的抗黄斑变性和抗肿瘤药物。丙二酰化/琥珀酰化 MLNs 更重要,因为它们具有克服多重耐药细菌的功效。然而,催化这种反应的酶仍然是个谜。在此,我们破译了负责此步骤的 β-内酰胺酶同源物 BmmI。BmmI 可以特异性地将 C3-C5 烷基酸硫酯连接到 MLN A 的 7-OH 上,并且还表现出对具有不同支架的酰基受体的底物混杂性。BmmI 与琥珀酰基共价连接的晶体结构和系统诱变突出了氧阴离子孔状几何形状在识别羧基端酰基供体中的作用。这种几何结构的工程扩大了其底物范围,R166A/G/Q 变体可识别多达 C12 烷基酸硫酯。BmmI 与酰基受体 MLN A 的结构揭示了 Arg292 在识别大环内酯底物方面的重要性。此外,建立了 BmmI 催化的酰基转移反应的机制,揭示了 Lys76 在控制酰基供体和催化方面的灵巧作用。我们的研究揭示了酰基转移酶底物选择性的微妙机制,这将指导药物开发的合理酶工程。
更新日期:2020-08-17
中文翻译:

细菌酰基转移酶中羧基末端酰基供体特异性的结构基础
Macrolactins (MLNs) 是一类重要的抗黄斑变性和抗肿瘤药物。丙二酰化/琥珀酰化 MLNs 更重要,因为它们具有克服多重耐药细菌的功效。然而,催化这种反应的酶仍然是个谜。在此,我们破译了负责此步骤的 β-内酰胺酶同源物 BmmI。BmmI 可以特异性地将 C3-C5 烷基酸硫酯连接到 MLN A 的 7-OH 上,并且还表现出对具有不同支架的酰基受体的底物混杂性。BmmI 与琥珀酰基共价连接的晶体结构和系统诱变突出了氧阴离子孔状几何形状在识别羧基端酰基供体中的作用。这种几何结构的工程扩大了其底物范围,R166A/G/Q 变体可识别多达 C12 烷基酸硫酯。BmmI 与酰基受体 MLN A 的结构揭示了 Arg292 在识别大环内酯底物方面的重要性。此外,建立了 BmmI 催化的酰基转移反应的机制,揭示了 Lys76 在控制酰基供体和催化方面的灵巧作用。我们的研究揭示了酰基转移酶底物选择性的微妙机制,这将指导药物开发的合理酶工程。































 京公网安备 11010802027423号
京公网安备 11010802027423号