Cellular and Molecular Gastroenterology and Hepatology ( IF 7.1 ) Pub Date : 2020-08-15 , DOI: 10.1016/j.jcmgh.2020.08.004 Ravi Chakra Turaga 1 , Malvika Sharma 1 , Falguni Mishra 1 , Alyssa Krasinskas 2 , Yi Yuan 1 , Jenny J Yang 3 , Shiyuan Wang 4 , Chunfeng Liu 4 , Sun Li 4 , Zhi-Ren Liu 1
|
Background & Aims
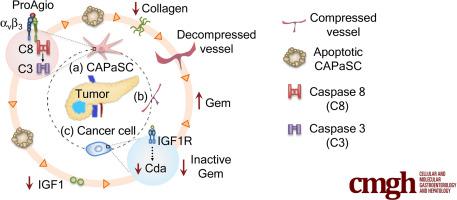
Pancreatic ductal adenocarcinoma (PDAC) is resistant to most therapeutics owing to dense fibrotic stroma orchestrated by cancer-associated pancreatic stellate cells (CAPaSC). CAPaSC also support cancer cell growth, metastasis, and resistance to apoptosis. Currently, there is no effective therapy for PDAC that specifically targets CAPaSC. We previously reported a rationally designed protein, ProAgio, that targets integrin αvβ3 at a novel site and induces apoptosis in integrin αvβ3-expressing cells. Because both CAPaSC and angiogenic endothelial cells express high levels of integrin αvβ3, we aimed to analyze the effects of ProAgio in PDAC tumor.
Methods
Expression of integrin αvβ3 was examined in both patient tissue and cultured cells. The effects of ProAgio on CAPaSC were analyzed using an apoptosis assay kit. The effects of ProAgio in PDAC tumor were studied in 3 murine tumor models: subcutaneous xenograft, genetic engineered (KrasG12D; p53R172H; Pdx1-Cre, GEM-KPC) mice, and an orthotopic KrasG12D; p53R172H; Pdx1-Cre (KPC) model.
Results
ProAgio induces apoptosis in CAPaSC. ProAgio treatment significantly prolonged survival of a genetically engineered mouse–KPC and orthotopic KPC mice alone or in combination with gemcitabine (Gem). ProAgio specifically induced apoptosis in CAPaSC, resorbed collagen, and opened collapsed tumor vessels without an increase in angiogenesis in PDAC tumor, enabling drug delivery into the tumor. ProAgio decreased intratumoral insulin-like growth factor 1 levels as a result of depletion of CAPaSC and consequently decreased cytidine deaminase, a Gem metabolism enzyme in cancer cells, and thereby reduced resistance to Gem-induced apoptosis.
Conclusions
Our study suggests that ProAgio is an effective PDAC treatment agent because it specifically depletes CAPaSC and eliminates tumor angiogenesis, thereby enhancing drug delivery and Gem efficacy in PDAC tumors.
中文翻译:

通过整合素 αvβ3 靶向蛋白调节癌症相关纤维化基质用于胰腺癌治疗。
背景与目标
由于癌症相关胰腺星状细胞 (CAPaSC) 精心编排的致密纤维化基质,胰腺导管腺癌 (PDAC) 对大多数治疗方法具有耐药性。 CAPaSC 还支持癌细胞生长、转移和抵抗细胞凋亡。目前,尚无专门针对 CAPaSC 的 PDAC 有效疗法。我们之前报道了一种合理设计的蛋白质 ProAgio,它在一个新位点靶向整合素 α v β 3并诱导表达整合素 α v β 3的细胞凋亡。由于 CAPaSC 和血管生成内皮细胞均表达高水平的整合素 α v β 3 ,因此我们旨在分析 ProAgio 在 PDAC 肿瘤中的作用。
方法
在患者组织和培养细胞中检查了整合素 α v β 3的表达。使用细胞凋亡检测试剂盒分析 ProAgio 对 CAPaSC 的影响。在 3 种小鼠肿瘤模型中研究了 ProAgio 在 PDAC 肿瘤中的作用:皮下异种移植、基因工程(Kras G12D ;p53 R172H ;Pdx1-Cre、GEM-KPC)小鼠和原位 KrasG12D; p53R172H; Pdx1-Cre (KPC) 模型。
结果
ProAgio 诱导 CAPaSC 细胞凋亡。 ProAgio 单独治疗或与吉西他滨 (Gem) 联合治疗可显着延长基因工程小鼠 KPC 和原位 KPC 小鼠的存活时间。 ProAgio 特异性诱导 CAPaSC 细胞凋亡、吸收胶原蛋白并打开塌陷的肿瘤血管,而不会增加 PDAC 肿瘤中的血管生成,从而能够将药物输送到肿瘤中。由于 CAPaSC 耗竭,ProAgio 降低了瘤内胰岛素样生长因子 1 水平,从而降低了胞苷脱氨酶(癌细胞中的一种 Gem 代谢酶),从而降低了对 Gem 诱导的细胞凋亡的抵抗力。
结论
我们的研究表明,ProAgio 是一种有效的 PDAC 治疗剂,因为它特异性地消耗 CAPaSC 并消除肿瘤血管生成,从而增强 PDAC 肿瘤中的药物输送和 Gem 功效。































 京公网安备 11010802027423号
京公网安备 11010802027423号