Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2020-08-15 , DOI: 10.1016/j.jssc.2020.121660 Debanjana Pahari , Samiran Misra , Partha P. Jana , Sreeraj Puravankara
|
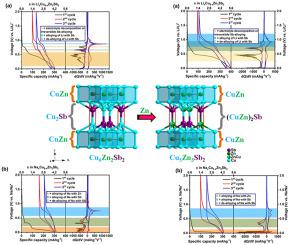
Ternary intermetallics with nominal composition Cu6-δZn2+δSb2 (δ = 0 and 1) are synthesized by high-temperature solid-state synthesis and characterized using powder X-ray diffraction. Electrochemical characterization of ternary intermetallic Cu6- δZn2+δSb2 (δ = 0 and 1) as a potential anode in Li-ion and Na-ion batteries is studied. Cu6Zn2Sb2 as a material for Li-ion storage shows an initial lithiation capacity of 300 mAhg−1, which falls to 200 mAhg−1 for the next delithiation cycle corresponding to dealloying of ~3 Li-ions. The ex-situ powder XRD studies at different stages of charging and discharging revealed Li3Sb alloy as the final product with no Li–Zn alloying during Li-ion insertion. The Na-ion insertion follows a different mechanism in Cu6Zn2Sb2, forming Na–Zn (NaZn13) and Na3Sb in the first and subsequent cycles. The first cycle capacity for Na alloying is found to be ~320 mAhg−1. The reaction mechanism for Li-ion and Na-ion alloying with Cu5Zn3Sb2 results in Li–Zn and NaZn13 alloy formation. The capacity retentions of Cu6Zn2Sb2 and Cu5Zn3Sb2 during Li-ion reaction are found to be ~36 mAhg−1 and ~109 mAhg−1 after 20 cycles at a C-rate of C/20. Cu5Zn3Sb2 electrodes show three times as much capacity retention in comparison with Cu6Zn2Sb2 electrodes as the Zn alloying reaction is found to be a contributing factor for capacity retention. The effect of additives during charge-discharge cycling of the ternary intermetallic is characterized using 2% and 5% Fluoroethylene Carbonate (FEC). The initial capacities improved for both Cu6Zn2Sb2 and Cu5Zn3Sb2, but the capacity retention properties improved only for the Cu6Zn2Sb2 for Li-alloying reactions.
中文翻译:

电化学合金化/脱合金的三元机制间的Cu 6-δ锌2 +δ的Sb 2(δ= 0和1)作为阳极为锂离子和Na离子电池
与三元金属间化合物标称组成的Cu 6-δ的Zn 2 +δ的Sb 2(δ= 0和1)由高温固态合成合成,并使用粉末X射线衍射来表征。的电化学表征三元金属间的Cu 6-δ锌2 +δ的Sb 2(δ= 0和1)作为在锂离子和Na离子电池的电势的阳极进行了研究。作为锂离子存储材料的Cu 6 Zn 2 Sb 2的初始锂化能力为300 mAhg -1,降至200 mAhg -1在下一个脱锂循环中,对应于约3个锂离子的脱合金。在充电和放电的不同阶段进行的异位粉末XRD研究表明,Li 3 Sb合金是最终产品,在锂离子插入过程中没有Li-Zn合金化。Na离子的插入在Cu 6 Zn 2 Sb 2中遵循不同的机理,在第一个循环及随后的循环中形成Na-Zn(NaZn 13)和Na 3 Sb。Na合金化的第一个循环容量为〜320 mAhg -1。锂离子和钠离子与Cu 5 Zn 3 Sb 2合金化的反应机理产生锂锌和钠锌13合金形成。在C / C速率下,经过20个循环后,锂离子反应期间Cu 6 Zn 2 Sb 2和Cu 5 Zn 3 Sb 2的容量保持率为〜36 mAhg -1和〜109 mAhg -1。 20 Cu 5 Zn 3 Sb 2电极的容量保持能力是Cu 6 Zn 2 Sb 2的三倍锌合金化反应的电极被发现是保持容量的一个重要因素。使用2%和5%氟代碳酸亚乙酯(FEC)来表征添加剂在三元金属间化合物的充放电循环中的作用。对于Cu 6 Zn 2 Sb 2和Cu 5 Zn 3 Sb 2,初始容量均得到改善,但是仅对于用于锂合金化反应的Cu 6 Zn 2 Sb 2,容量保持性质得以改善。































 京公网安备 11010802027423号
京公网安备 11010802027423号