当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Helic[1]triptycene[3]arene: Synthesis, Complexation, and Formation of [2]Rotaxane Shuttle.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-08-14 , DOI: 10.1021/acs.joc.0c01558 Jing Li 1, 2 , Xiao-Ni Han 1, 2 , He-Ye Zhou 1, 2 , Ying Han 1 , Chuan-Feng Chen 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-08-14 , DOI: 10.1021/acs.joc.0c01558 Jing Li 1, 2 , Xiao-Ni Han 1, 2 , He-Ye Zhou 1, 2 , Ying Han 1 , Chuan-Feng Chen 1, 2
Affiliation
|
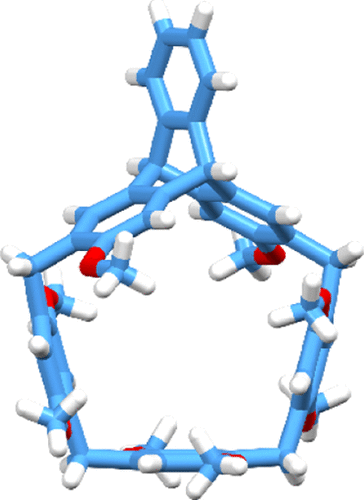
A new macrocyclic arene, helic[1]triptycene[3]arene H, was conveniently synthesized in 37% yield by a one-pot reaction starting from 2,6-dimethoxyl-3,7-dihydroxymethyltriptycene. Macrocycle H showed fixed conformation in solution and could form 1:1 complexes with a series of neutral guests, secondary ammonium salts, and tertiary ammonium salts in both solution and solid states. The association constants between H and the neutral guests were between (1.23 ± 0.10) × 102 and (4.70 ± 0.47) × 103 M–1, while the association constants between H and the ammonium guests were between (1.35 ± 0.12) × 103 and (1.59 ± 0.14) × 105 M–1. Moreover, H showed bigger association constants with secondary ammonium salts than those with tertiary ammonium salts possibly because of the steric hindrance effect and multiple intermolecular interactions. The stimuli-responsive complexation between H and the ammonium salts could be controlled by the addition and removal of acids and bases as well. Based on the host–guest complexation between H and the secondary ammonium salt, [2]rotaxane was further synthesized, and its shuttling motion could be efficiently controlled by an acid and base.
中文翻译:

螺旋[1]三茂[3]芳烃:[2]轮烷的穿梭反应的合成,络合和形成。
通过从2,6-二甲氧基-1,3,7-二羟基甲基三茂苯开始的一锅反应,以37%的收率方便地合成了一种新的大环芳烃,螺旋[1]三茂[3]芳烃H。大环H在溶液中显示出固定的构象,并可以与一系列中性客体,仲铵盐和叔铵盐在溶液和固态下形成1:1的络合物。H和中性客体之间的缔合常数在(1.23±0.10)×10 2和(4.70±0.47)×10 3 M –1之间,而H和铵客体之间的缔合常数在(1.35±0.12)× 10 3和(1.59±0.14)×10 5M –1。此外,H显示出与仲铵盐相比具有比叔铵盐更大的缔合常数,这可能是由于空间位阻效应和多种分子间相互作用。H和铵盐之间的刺激响应络合物也可以通过添加和去除酸和碱来控制。基于H和仲铵盐之间的主客体络合作用,进一步合成了[2]轮烷,其穿梭运动可以通过酸和碱有效控制。
更新日期:2020-09-05
中文翻译:

螺旋[1]三茂[3]芳烃:[2]轮烷的穿梭反应的合成,络合和形成。
通过从2,6-二甲氧基-1,3,7-二羟基甲基三茂苯开始的一锅反应,以37%的收率方便地合成了一种新的大环芳烃,螺旋[1]三茂[3]芳烃H。大环H在溶液中显示出固定的构象,并可以与一系列中性客体,仲铵盐和叔铵盐在溶液和固态下形成1:1的络合物。H和中性客体之间的缔合常数在(1.23±0.10)×10 2和(4.70±0.47)×10 3 M –1之间,而H和铵客体之间的缔合常数在(1.35±0.12)× 10 3和(1.59±0.14)×10 5M –1。此外,H显示出与仲铵盐相比具有比叔铵盐更大的缔合常数,这可能是由于空间位阻效应和多种分子间相互作用。H和铵盐之间的刺激响应络合物也可以通过添加和去除酸和碱来控制。基于H和仲铵盐之间的主客体络合作用,进一步合成了[2]轮烷,其穿梭运动可以通过酸和碱有效控制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号