当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dehydroxylative Fluorination of Tertiary Alcohols.
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.orglett.0c02438
Wei Zhang 1 , Yu-Cheng Gu 2 , Jin-Hong Lin 1 , Ji-Chang Xiao 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.orglett.0c02438
Wei Zhang 1 , Yu-Cheng Gu 2 , Jin-Hong Lin 1 , Ji-Chang Xiao 1
Affiliation
|
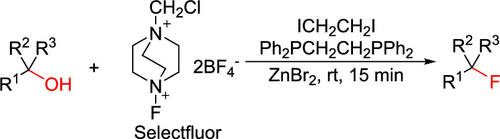
A large number of fluorination methods have been developed, but the construction of a tertiary C–F bond remains challenging. Herein, we describe an efficient dehydroxylative fluorination of tertiary alcohols with Selectfluor via the activation of a hydroxyl group by a Ph2PCH2CH2PPh2/ICH2CH2I system. Although the reagents appear to be not compatible (Selectfluor with the phosphine and I– generated in situ), the reactions occur rapidly to give the desired products in moderate to high yields. This work may present a new discovery in fluorination of alcohols since the reported methods are mainly limited to primary and secondary alcohols.
中文翻译:

叔醇的脱羟基氟化。
已经开发出了许多氟化方法,但是三次C-F键的构建仍然具有挑战性。在本文中,我们描述了通过Ph 2 PCH 2 CH 2 PPh 2 / ICH 2 CH 2 I系统活化羟基,用Selectfluor有效地进行叔醇的脱羟基氟化反应。虽然试剂似乎是不兼容(的Selectfluor与膦和我-原位产生),快速地发生反应,得到所期望的产物在较高产率。由于报道的方法主要限于伯醇和仲醇,因此这项工作可能会在醇的氟化方面提出新的发现。
更新日期:2020-08-21
中文翻译:

叔醇的脱羟基氟化。
已经开发出了许多氟化方法,但是三次C-F键的构建仍然具有挑战性。在本文中,我们描述了通过Ph 2 PCH 2 CH 2 PPh 2 / ICH 2 CH 2 I系统活化羟基,用Selectfluor有效地进行叔醇的脱羟基氟化反应。虽然试剂似乎是不兼容(的Selectfluor与膦和我-原位产生),快速地发生反应,得到所期望的产物在较高产率。由于报道的方法主要限于伯醇和仲醇,因此这项工作可能会在醇的氟化方面提出新的发现。

































 京公网安备 11010802027423号
京公网安备 11010802027423号