当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Activity Relationship Study of T1R2/T1R3 Positive Allosteric Modulators and Evaluation of Their Enhancing Effect on Various Sweeteners
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-08-13 , DOI: 10.1002/slct.202002159 Ryo Matsumoto 1 , Kei Yamada 1 , Masakazu Nakazawa 1 , Suguru Mori 1 , Seiji Kitajima 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-08-13 , DOI: 10.1002/slct.202002159 Ryo Matsumoto 1 , Kei Yamada 1 , Masakazu Nakazawa 1 , Suguru Mori 1 , Seiji Kitajima 1
Affiliation
|
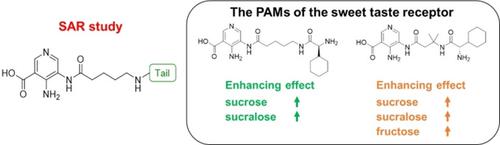
The sweet taste receptor T1R2/T1R3 belongs to the family of G protein coupled receptors and recognizes various sweet substances from small compounds to proteins. We have previously reported unnatural tripeptides that function as positive allosteric modulators (PAMs) of the T1R2/T1R3. The structures were classified into three parts—head, linker, and tail—as compared with known T1R2/T1R3 PAMs. Here, we conducted a structure–activity relationship study of the tail structure to further understand the structural features of the T1R2/T1R3 PAMs. Moreover, we evaluated the enhancing effect of these PAMs on various sweeteners. In conclusion, we found that both hydrophilic α‐amino acids and the hydrophobicity of the α‐amino acid side chain are important for enhancing activity for this receptor. These PAMs also showed the receptor enhancing activity against sugar‐like T1R2/T1R3 agonists such as sucrose, sucralose, and fructose.
中文翻译:

T1R2 / T1R3正构构调节剂的构效关系研究及其对各种甜味剂的增强作用
甜味受体T1R2 / T1R3属于G蛋白偶联受体家族,可识别从小化合物到蛋白质的各种甜味物质。我们以前曾报道过非天然的三肽,它们充当T1R2 / T1R3的正变构调节剂(PAM)。与已知的T1R2 / T1R3 PAM相比,结构分为头部,连接器和尾部三部分。在这里,我们进行了尾部结构的构效关系研究,以进一步了解T1R2 / T1R3 PAM的结构特征。此外,我们评估了这些PAM对各种甜味剂的增强作用。总之,我们发现亲水性α-氨基酸和α-氨基酸侧链的疏水性对于增强该受体的活性都很重要。
更新日期:2020-08-14
中文翻译:

T1R2 / T1R3正构构调节剂的构效关系研究及其对各种甜味剂的增强作用
甜味受体T1R2 / T1R3属于G蛋白偶联受体家族,可识别从小化合物到蛋白质的各种甜味物质。我们以前曾报道过非天然的三肽,它们充当T1R2 / T1R3的正变构调节剂(PAM)。与已知的T1R2 / T1R3 PAM相比,结构分为头部,连接器和尾部三部分。在这里,我们进行了尾部结构的构效关系研究,以进一步了解T1R2 / T1R3 PAM的结构特征。此外,我们评估了这些PAM对各种甜味剂的增强作用。总之,我们发现亲水性α-氨基酸和α-氨基酸侧链的疏水性对于增强该受体的活性都很重要。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号