当前位置:
X-MOL 学术
›
Redox Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytosolic ME1 integrated with mitochondrial IDH2 supports tumor growth and metastasis
Redox Biology ( IF 10.7 ) Pub Date : 2020-08-13 , DOI: 10.1016/j.redox.2020.101685
Chang Shao 1 , Wenjie Lu 2 , Ye Du 3 , Wenchao Yan 2 , Qiuyu Bao 2 , Yang Tian 2 , Guangji Wang 4 , Hui Ye 5 , Haiping Hao 1
Redox Biology ( IF 10.7 ) Pub Date : 2020-08-13 , DOI: 10.1016/j.redox.2020.101685
Chang Shao 1 , Wenjie Lu 2 , Ye Du 3 , Wenchao Yan 2 , Qiuyu Bao 2 , Yang Tian 2 , Guangji Wang 4 , Hui Ye 5 , Haiping Hao 1
Affiliation
|
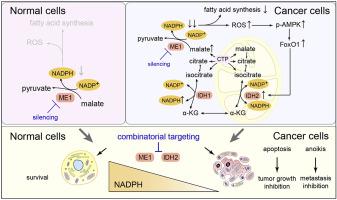
NADPH is a pivotal cofactor that maintains redox homeostasis and lipogenesis in cancer cells and interference with NADPH production is a promising approach for treating cancer. However, how normal and cancer cells differentially exploit NADPH-producing pathways is unclear, and selective approaches to targeting NADPH are lacking. Here, we show that the assayed cancer cell lines preferentially depend on ME1-mediated NADPH production. ME1 knockdown increases intracellular ROS levels and impairs lipogenesis in cancer cells, leading to retarded proliferation and increased anoikis, while sparing normal cells. Notably, ME1 interference ultimately resulted in adaptive upregulation of mitochondrial IDH2 dependent of AMPK-FoxO1 activation to replenish the NADPH pool and mitigate cytosolic ROS. Combining ME1 ablation and IDH2 inhibition drastically reduces intracellular NADPH and prevents resistance to ME1 interference, resulting in increased apoptosis and impeded tumor growth and metastasis. This study demonstrates that cytosolic ME1 integrated with mitochondrial IDH2 is essential for tumor growth and metastasis, thereby highlighting the blockade of metabolic compensation by disrupting mitochondrial-cytosol NADPH transport as a promising approach to selectively targeting NADPH in cancer cells that rely on NADPH-driven antioxidant systems.
中文翻译:

胞浆 ME1 与线粒体 IDH2 整合支持肿瘤生长和转移
NADPH 是维持癌细胞氧化还原稳态和脂肪生成的关键辅助因子,干扰 NADPH 的产生是治疗癌症的一种有前景的方法。然而,正常细胞和癌细胞如何差异化利用 NADPH 产生途径尚不清楚,并且缺乏针对 NADPH 的选择性方法。在这里,我们表明所检测的癌细胞系优先依赖于 ME1 介导的 NADPH 产生。 ME1 敲低会增加细胞内 ROS 水平并损害癌细胞的脂肪生成,导致增殖迟缓和失巢凋亡增加,同时不影响正常细胞。值得注意的是,ME1 干扰最终导致依赖于 AMPK-FoxO1 激活的线粒体 IDH2 适应性上调,以补充 NADPH 池并减少胞质 ROS。将 ME1 消除和 IDH2 抑制相结合,可大幅降低细胞内 NADPH 并防止对 ME1 干扰的抵抗,从而导致细胞凋亡增加并阻碍肿瘤生长和转移。这项研究表明,胞质 ME1 与线粒体 IDH2 整合对于肿瘤生长和转移至关重要,从而强调通过破坏线粒体 - 胞质 NADPH 运输来阻断代谢补偿是一种有前途的方法,可以在依赖 NADPH 驱动的抗氧化剂的癌细胞中选择性靶向 NADPH系统。
更新日期:2020-08-13
中文翻译:

胞浆 ME1 与线粒体 IDH2 整合支持肿瘤生长和转移
NADPH 是维持癌细胞氧化还原稳态和脂肪生成的关键辅助因子,干扰 NADPH 的产生是治疗癌症的一种有前景的方法。然而,正常细胞和癌细胞如何差异化利用 NADPH 产生途径尚不清楚,并且缺乏针对 NADPH 的选择性方法。在这里,我们表明所检测的癌细胞系优先依赖于 ME1 介导的 NADPH 产生。 ME1 敲低会增加细胞内 ROS 水平并损害癌细胞的脂肪生成,导致增殖迟缓和失巢凋亡增加,同时不影响正常细胞。值得注意的是,ME1 干扰最终导致依赖于 AMPK-FoxO1 激活的线粒体 IDH2 适应性上调,以补充 NADPH 池并减少胞质 ROS。将 ME1 消除和 IDH2 抑制相结合,可大幅降低细胞内 NADPH 并防止对 ME1 干扰的抵抗,从而导致细胞凋亡增加并阻碍肿瘤生长和转移。这项研究表明,胞质 ME1 与线粒体 IDH2 整合对于肿瘤生长和转移至关重要,从而强调通过破坏线粒体 - 胞质 NADPH 运输来阻断代谢补偿是一种有前途的方法,可以在依赖 NADPH 驱动的抗氧化剂的癌细胞中选择性靶向 NADPH系统。

































 京公网安备 11010802027423号
京公网安备 11010802027423号