European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-08-14 , DOI: 10.1016/j.ejmech.2020.112703 Shulei Pan 1 , Yangli Zhou 1 , Qiusheng Wang 1 , Yanlin Wang 1 , Chenyu Tian 1 , Tianqi Wang 1 , Luyi Huang 1 , Jinshan Nan 1 , Linli Li 2 , Shengyong Yang 1
|
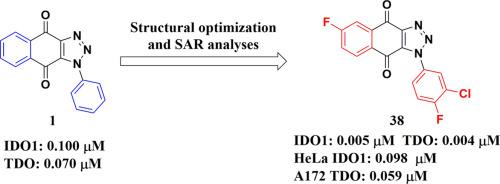
Indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophan 2,3-dioxygenase (TDO), which mediate kynurenine pathway of tryptophan degradation, have emerged as potential new targets in immunotherapy for treatment of cancer because of their critical role in immunosuppression in the tumor microenvironment. In this investigation, we report the structural optimization and structure-activity relationship studies of 1-phenyl-1H-naphtho[2,3-d][1,2,3]triazole-4,9-dione derivatives as a new class of IDO1/TDO dual inhibitors. Among all the obtained dual inhibitors, 1-(3-chloro-4-fluorophenyl)-6-fluoro-1H-naphtho[2,3-d][1,2,3]triazole-4,9-dione (38) displayed the most potent IDO1 and TDO inhibitory activities with IC50 (half-maximal inhibitory concentration) values of 5 nM for IDO1 and 4 nM for TDO. It turned out that compound 38 was not a PAINS compound. Compound 38 could efficiently inhibit the biofunction of IDO1 and TDO in intact cells. In LL2 (Lewis lung cancer) and Hepa1-6 (hepatic carcinoma) allograft mouse models, this compound also showed considerable in vivo anti-tumor activity and no obvious toxicity was observed. Therefore, 38 could be a good lead compound for cancer immunotherapy and deserving further investigation.
中文翻译:

1-芳基-1H-萘并[2,3-d][1,2,3]三唑-4,9-二酮衍生物作为吲哚胺2,3-双加氧酶1的有效双重抑制剂的发现和构效关系研究( IDO1) 和色氨酸 2,3-双加氧酶 (TDO)。
介导色氨酸降解犬尿氨酸途径的吲哚胺 2,3-双加氧酶 1 (IDO1) 和色氨酸 2,3-双加氧酶 (TDO) 已成为治疗癌症的免疫疗法的潜在新靶点,因为它们在免疫抑制中的关键作用肿瘤微环境。在这项研究中,我们报告了 1-苯基-1 H-萘并[2,3- d ][1,2,3]三唑-4,9-二酮衍生物的结构优化和构效关系研究作为一个新类别IDO1/TDO双重抑制剂。在得到的所有双重抑制剂中,1-(3-chloro-4-fluorophenyl)-6-fluoro-1 H - naphtho[2,3- d ][1,2,3]triazole-4,9-dione ( 38 )显示出最有效的 IDO1 和 TDO 抑制活性与 IC50(半最大抑制浓度)值,IDO1 为 5 nM,TDO 为 4 nM。结果表明,化合物38不是 PAINS 化合物。化合物38可以有效抑制完整细胞中IDO1和TDO的生物功能。在 LL2(Lewis 肺癌)和 Hepa1-6(肝癌)同种异体移植小鼠模型中,该化合物也显示出相当大的体内抗肿瘤活性,未观察到明显的毒性。因此,38可能是一种很好的癌症免疫治疗先导化合物,值得进一步研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号