Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-08-13 , DOI: 10.1016/j.apsb.2020.08.002 Yinqian Yang , Yongjiu Lv , Chengying Shen , Tingting Shi , Haisheng He , Jianping Qi , Xiaochun Dong , Weili Zhao , Yi Lu , Wei Wu
|
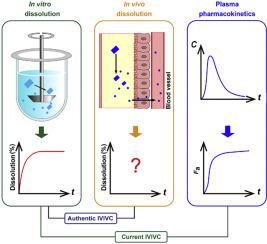
In vitro‒in vivo correlation (IVIVC) of solid dosage forms should be established basically between in vitro and in vivo dissolution of active pharmaceutical ingredients. Nevertheless, in vivo dissolution profiles have never been accurately portrayed. The current practice of IVIVC has to resort to in vivo absorption fractions (Fa). In this proof-of-concept study, in vivo dissolution of a model poorly water-soluble drug fenofibrate (FNB) was investigated by fluorescence bioimaging. FNB crystals were first labeled by near-infrared fluorophores with aggregation-caused quenching properties. The dyes illuminated FNB crystals but quenched immediately and absolutely once been released into aqueous media, enabling accurate monitoring of residual drug crystals. The linearity established between fluorescence and crystal concentration justified reliable quantification of FNB crystals. In vitro dissolution was first measured following pharmacopoeia monograph protocols with well-documented IVIVC. The synchronicity between fluorescence and in vitro dissolution of FNB supported using fluorescence as a measure for determination of dissolution. In vitro dissolution correlated well with in vivo dissolution, acquired by either live or ex vivo imaging. The newly established IVIVC was further validated by correlating both in vitro and in vivo dissolution with Fa obtained from pharmacokinetic data.
中文翻译:

水溶性差的药物在体内的溶解:基于荧光生物成像的概念验证
固体剂型的体外-体内相关性(IVIVC)应基本在活性药物成分的体外和体内溶出之间建立。然而,从未准确地描绘出体内溶出曲线。IVIVC的当前实践必须求助于体内吸收分数(F a)。在这项概念验证研究中,体内通过荧光生物成像研究了水溶性差的非诺贝特药物(FNB)的模型溶解。首先用具有聚集引起的猝灭特性的近红外荧光团标记FNB晶体。染料可以照亮FNB晶体,但是一旦被释放到水性介质中就立即被淬灭,从而能够精确监控残留的药物晶体。在荧光和晶体浓度之间建立的线性证明了FNB晶体的可靠定量。首先按照药典专论的规程并使用有据可查的IVIVC测定体外溶出度。荧光与FNB体外溶解之间的同步性支持使用荧光作为测定溶解度的手段。体外溶出度与通过实时或离体成像获得的体内溶出度密切相关。通过将体外和体内溶出度与从药代动力学数据获得的F a相关联,进一步验证了新建立的IVIVC 。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号