当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conjugation Site Influences Antibody-Conjugated Drug PK Assays: Case Studies for Disulfide-Linked, Self-Immolating Next-Generation Antibody Drug Conjugates.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.analchem.0c00773 M Violet Lee 1 , Surinder Kaur 1 , Ola M Saad 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.analchem.0c00773 M Violet Lee 1 , Surinder Kaur 1 , Ola M Saad 1
Affiliation
|
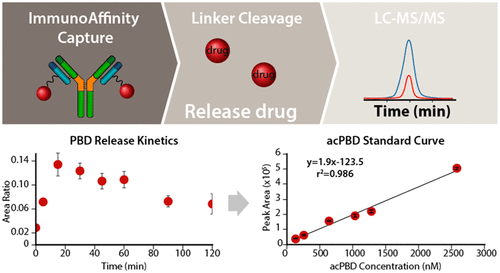
Immunoaffinity (IA) LC-MS/MS pharmacokinetic (PK) assays are widely used in the field for antibody drug conjugates (ADCs) containing peptide linkers that are enzymatically cleavable, such as MC-ValCit-PAB. Conjugate PK assay strategies for these ADCs involve cleavage with cathepsin B or papain to release and measure the antibody-conjugated drug (acDrug) concentration. However, robust acDrug PK methods for disulfide-linked self-immolating ADCs are lacking as they are a different conjugation modality. We developed acDrug PK assays for next-generation disulfide-linked ADCs involving immunoaffinity capture, chemical cleavage, and LC-MS/MS. Disulfide-linked ADCs captured from plasma were chemically reduced at basic pH to release the linker-drug, followed by self-immolation to liberate the active drug, and quantified by MRM LC-MS/MS. Herein, we detail the development and optimization of this chemical cleavage acDrug PK assay, resulting in robust accuracy and precision (±20%). The conjugation site of the linker-drug on the antibody was found to affect the kinetics of drug release. Multiple biophysical and chemical characteristics, such as tertiary structure, fractional solvent accessibility, pKa of the conjugation site, surrounding residue’s pI, and electrostatic charge, may directly impact the drug release kinetics. Similar site-specific stability has been previously reported for ADCs in vivo. The assay development and qualification data for this original assay format are presented along with its application to multiple in vitro and in vivo studies across species.
中文翻译:

结合位点影响抗体结合的药物PK分析:二硫键连接的,自消灭的下一代抗体药物结合物的案例研究。
免疫亲和(IA)LC-MS / MS药代动力学(PK)分析已广泛用于包含可酶切的肽接头(例如MC-ValCit-PAB)的抗体药物偶联物(ADC)。这些ADC的共轭PK测定策略涉及用组织蛋白酶B或木瓜蛋白酶裂解,以释放和测量抗体结合药物(acDrug)的浓度。但是,缺少用于二硫键连接的自消旋ADC的可靠acDrug PK方法,因为它们是不同的共轭形式。我们为涉及免疫亲和捕获,化学裂解和LC-MS / MS的下一代二硫键连接的ADC开发了acDrug PK分析。从血浆中捕获的二硫键连接的ADC在碱性pH值下化学还原以释放连接剂-药物,然后自焚以释放活性药物,并通过MRM LC-MS / MS进行定量。在这里 我们详细介绍了这种化学裂解acDrug PK检测方法的开发和优化,可实现稳定的准确性和精密度(±20%)。发现抗体上的接头药物的缀合位点影响药物释放的动力学。多种生物物理和化学特性,例如三级结构,部分溶剂可及性,pķ一个共轭部位,周围残留的PI和静电电荷,可以直接影响到药物的释放动力学。先前已报道过体内ADC具有相似的位点特异性稳定性。介绍了此原始测定格式的测定开发和鉴定数据,并将其应用于跨物种的多个体外和体内研究。
更新日期:2020-09-15
中文翻译:

结合位点影响抗体结合的药物PK分析:二硫键连接的,自消灭的下一代抗体药物结合物的案例研究。
免疫亲和(IA)LC-MS / MS药代动力学(PK)分析已广泛用于包含可酶切的肽接头(例如MC-ValCit-PAB)的抗体药物偶联物(ADC)。这些ADC的共轭PK测定策略涉及用组织蛋白酶B或木瓜蛋白酶裂解,以释放和测量抗体结合药物(acDrug)的浓度。但是,缺少用于二硫键连接的自消旋ADC的可靠acDrug PK方法,因为它们是不同的共轭形式。我们为涉及免疫亲和捕获,化学裂解和LC-MS / MS的下一代二硫键连接的ADC开发了acDrug PK分析。从血浆中捕获的二硫键连接的ADC在碱性pH值下化学还原以释放连接剂-药物,然后自焚以释放活性药物,并通过MRM LC-MS / MS进行定量。在这里 我们详细介绍了这种化学裂解acDrug PK检测方法的开发和优化,可实现稳定的准确性和精密度(±20%)。发现抗体上的接头药物的缀合位点影响药物释放的动力学。多种生物物理和化学特性,例如三级结构,部分溶剂可及性,pķ一个共轭部位,周围残留的PI和静电电荷,可以直接影响到药物的释放动力学。先前已报道过体内ADC具有相似的位点特异性稳定性。介绍了此原始测定格式的测定开发和鉴定数据,并将其应用于跨物种的多个体外和体内研究。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号