当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Dehydrogenation of N-Heterocycles Using Molecular Oxygen.
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.orglett.0c02271
Sourajit Bera 1 , Atanu Bera 1 , Debasis Banerjee 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.orglett.0c02271
Sourajit Bera 1 , Atanu Bera 1 , Debasis Banerjee 1
Affiliation
|
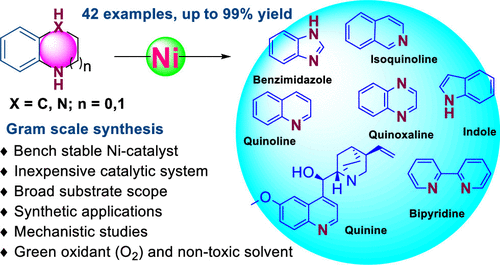
Herein, an efficient and selective nickel-catalyzed dehydrogenation of five- and six-membered N-heterocycles is presented. The transformation occurs in the presence of alkyl, alkoxy, chloro, free hydroxyl and primary amine, internal and terminal olefin, trifluoromethyl, and ester functional groups. Synthesis of an important ligand and the antimalarial drug quinine is demonstrated. Mechanistic studies revealed that the cyclic imine serves as the key intermediate for this stepwise transformation.
中文翻译:

使用分子氧对N-杂环进行镍催化的脱氢
本文中,提出了五元和六元N-杂环的有效且选择性的镍催化的脱氢。该转化在烷基,烷氧基,氯,游离羟基和伯胺,内部和末端烯烃,三氟甲基和酯官能团的存在下发生。证明了重要配体和抗疟药奎宁的合成。机理研究表明,环状亚胺是该逐步转化的关键中间体。
更新日期:2020-08-21
中文翻译:

使用分子氧对N-杂环进行镍催化的脱氢
本文中,提出了五元和六元N-杂环的有效且选择性的镍催化的脱氢。该转化在烷基,烷氧基,氯,游离羟基和伯胺,内部和末端烯烃,三氟甲基和酯官能团的存在下发生。证明了重要配体和抗疟药奎宁的合成。机理研究表明,环状亚胺是该逐步转化的关键中间体。































 京公网安备 11010802027423号
京公网安备 11010802027423号