当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Halide-Accelerated Acyl Fluoride Formation Using Sulfuryl Fluoride.
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.orglett.0c02566 Paul J Foth 1 , Thomas C Malig 1 , Hao Yu 1 , Trevor G Bolduc 1 , Jason E Hein 1 , Glenn M Sammis 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.orglett.0c02566 Paul J Foth 1 , Thomas C Malig 1 , Hao Yu 1 , Trevor G Bolduc 1 , Jason E Hein 1 , Glenn M Sammis 1
Affiliation
|
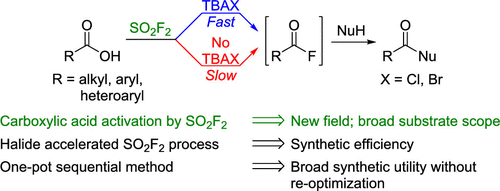
Herein, we report a new one-pot sequential method for SO2F2-mediated nucleophilic acyl substitution reactions starting from carboxylic acids. A mechanistic study revealed that SO2F2-mediated acid activation proceeds via the anhydride, which is then converted to the corresponding acyl fluoride. Tetrabutylammonium chloride or bromide accelerate the formation of acyl fluoride. Optimized halide-accelerated conditions were used to synthesize acyl fluorides in 30–80% yields, and esters, amides, and thioesters in 72–96% yields without reoptimization for each nucleophile.
中文翻译:

使用硫酰氟进行卤化物加速的酰氟形成。
在这里,我们报道了一种新的一锅法,用于从羧酸开始的SO 2 F 2介导的亲核酰基取代反应。机理研究表明,SO 2 F 2介导的酸活化是通过酸酐进行的,然后将其转化为相应的酰基氟。四丁基氯化铵或溴化物可加速酰基氟的形成。优化的卤化物加速条件可用于以30-80%的产率合成酰氟,而酯,酰胺和硫代酯的产率为72-96%,而无需对每个亲核试剂进行重新优化。
更新日期:2020-08-21
中文翻译:

使用硫酰氟进行卤化物加速的酰氟形成。
在这里,我们报道了一种新的一锅法,用于从羧酸开始的SO 2 F 2介导的亲核酰基取代反应。机理研究表明,SO 2 F 2介导的酸活化是通过酸酐进行的,然后将其转化为相应的酰基氟。四丁基氯化铵或溴化物可加速酰基氟的形成。优化的卤化物加速条件可用于以30-80%的产率合成酰氟,而酯,酰胺和硫代酯的产率为72-96%,而无需对每个亲核试剂进行重新优化。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号