当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of novel 4,5‐dihydropyrrolo[1,2‐ a ]quinoxalines, pyrrolo[1,2‐ a ]quinoxalin]‐2‐ones and their antituberculosis and anticancer activity
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-08-12 , DOI: 10.1002/ardp.202000192 Vitthal B Makane 1, 2 , Eruva Vamshi Krishna 3 , Uattam B Karale 1, 2 , Dattatraya A Babar 1, 2 , Saradhi Kalari 1, 2 , Estharla M Rekha 4 , Manjulika Shukla 5 , Grace Kaul 5 , Dharmarajan Sriram 4 , Sidharth Chopra 5 , Sunil Misra 2, 3 , Haridas B Rode 1, 2
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-08-12 , DOI: 10.1002/ardp.202000192 Vitthal B Makane 1, 2 , Eruva Vamshi Krishna 3 , Uattam B Karale 1, 2 , Dattatraya A Babar 1, 2 , Saradhi Kalari 1, 2 , Estharla M Rekha 4 , Manjulika Shukla 5 , Grace Kaul 5 , Dharmarajan Sriram 4 , Sidharth Chopra 5 , Sunil Misra 2, 3 , Haridas B Rode 1, 2
Affiliation
|
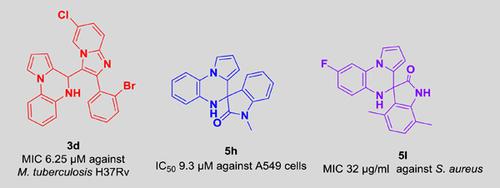
A facile strategy was developed for the synthesis of biologically important 4,5‐dihydropyrrolo[1,2‐a]quinoxalines and pyrrolo[1,2‐a]quinoxalin]‐2‐ones by treating 2‐(1H‐pyrrol‐1‐yl)anilines with imidazo[1,2‐a]pyridine‐3‐carbaldehyde or isatin, using amidosulfonic acid (NH3SO3) as a solid catalyst in water at room temperature. The protocol has been extended to electrophile ninhydrin. The catalyst could be recycled for six times without the loss of activity. The compounds were evaluated for their antituberculosis, antibacterial, and anticancer activities. It is worth noting that compounds 3d and 3e demonstrated a minimum inhibitory concentration value of 6.25 µM against Mycobacterium tuberculosis H37Rv, whereas compounds 3d, 3g, 5d, 5e, and 5i showed a remarkable inhibition of A549, DU145, HeLa, HepG2, MCF‐7, and B16‐F10 cell lines, respectively. Staphylococcus aureus was inhibited by compounds 5b, 5e, 5d, 5g, and 5l at 32 µg/ml.
中文翻译:

新型4,5-二氢吡咯并[1,2-a]喹喔啉、吡咯并[1,2-a]喹喔啉]-2-酮的合成及其抗结核和抗癌活性
通过处理 2-(1H-pyrrol-1-基)苯胺与咪唑并[1,2-a]吡啶-3-甲醛或靛红,在室温下使用酰胺磺酸(NH3SO3)作为固体催化剂在水中。该协议已扩展到亲电茚三酮。催化剂可循环使用六次而不会损失活性。评估了这些化合物的抗结核、抗菌和抗癌活性。值得注意的是,化合物 3d 和 3e 对结核分枝杆菌 H37Rv 的最小抑制浓度值为 6.25 µM,而化合物 3d、3g、5d、5e 和 5i 对 A549、DU145、HeLa、HepG2、MCF- 7 和 B16-F10 细胞系,分别。
更新日期:2020-08-12
中文翻译:

新型4,5-二氢吡咯并[1,2-a]喹喔啉、吡咯并[1,2-a]喹喔啉]-2-酮的合成及其抗结核和抗癌活性
通过处理 2-(1H-pyrrol-1-基)苯胺与咪唑并[1,2-a]吡啶-3-甲醛或靛红,在室温下使用酰胺磺酸(NH3SO3)作为固体催化剂在水中。该协议已扩展到亲电茚三酮。催化剂可循环使用六次而不会损失活性。评估了这些化合物的抗结核、抗菌和抗癌活性。值得注意的是,化合物 3d 和 3e 对结核分枝杆菌 H37Rv 的最小抑制浓度值为 6.25 µM,而化合物 3d、3g、5d、5e 和 5i 对 A549、DU145、HeLa、HepG2、MCF- 7 和 B16-F10 细胞系,分别。






























 京公网安备 11010802027423号
京公网安备 11010802027423号