当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spin-Related Electron Transfer and Orbital Interactions in Oxygen Electrocatalysis.
Advanced Materials ( IF 27.4 ) Pub Date : 2020-08-09 , DOI: 10.1002/adma.202003297
Yuanmiao Sun 1 , Shengnan Sun 1, 2 , Haitao Yang 3 , Shibo Xi 4 , Jose Gracia 5 , Zhichuan J Xu 1, 6
Advanced Materials ( IF 27.4 ) Pub Date : 2020-08-09 , DOI: 10.1002/adma.202003297
Yuanmiao Sun 1 , Shengnan Sun 1, 2 , Haitao Yang 3 , Shibo Xi 4 , Jose Gracia 5 , Zhichuan J Xu 1, 6
Affiliation
|
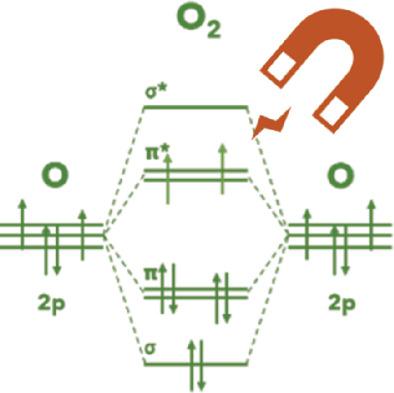
Oxygen evolution and reduction reactions play a critical role in determining the efficiency of the water cycling (H2O ⇔ H2 + O2), in which the hydrogen serves as the energy carrier. That calls for a comprehensive understanding of oxygen electrocatalysis for efficient catalyst design. Current opinions on oxygen electrocatalysis have been focused on the thermodynamics of the reactant/intermediate adsorption on the catalysts. Because the oxygen molecule is paramagnetic, its production from or its reduction to diamagnetic hydroxide/water involves spin‐related electron transfer. Both electron transfer and orbital interactions between the catalyst and the reactant/intermediate show spin‐dependent character, making the reaction kinetics and thermodynamics sensitive to the spin configurations. Herein, a brief introduction on the spintronic explanation of the catalytic phenomena on oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) is given. The local spin configurations and orbital interactions in the benchmark transition‐metal‐based catalysts for OER and ORR are analyzed as examples. To further understand the spintronic oxygen electrocatalysis and to develop more efficient spintronic catalysts, the challenges are summarized and future opportunities proposed. Spin electrocatalysis may emerge as an important topic in the near future and help integrate a comprehensive understanding of oxygen electrocatalysis.
中文翻译:

自旋相关的电子转移和氧电催化中的轨道相互作用。
氧气的释放和还原反应在决定水循环的效率(H 2 O⇔H 2 +Ø 2),其中氢用作能量载体。这就要求对氧电催化法有一个全面的了解,以进行有效的催化剂设计。关于氧电催化的当前观点集中在反应物/催化剂上的中间吸附的热力学上。因为氧分子是顺磁性的,所以其从反磁性氢氧化物/水中产生或还原成反磁性氢氧化物/水都涉及自旋相关的电子转移。催化剂与反应物/中间体之间的电子转移和轨道相互作用都显示出自旋依赖性,使反应动力学和热力学对自旋构型敏感。在此,简要介绍了自旋电子学,以解释氧释放反应(OER)和氧还原反应(ORR)的催化现象。以OER和ORR为基准的过渡金属基催化剂中的局部自旋构型和轨道相互作用为例进行了分析。为了进一步了解自旋电子氧电催化作用并开发出更有效的自旋电子催化剂,总结了挑战并提出了未来的机会。自旋电催化在不久的将来可能会成为一个重要的话题,并有助于整合对氧电催化的全面理解。
更新日期:2020-10-02
中文翻译:

自旋相关的电子转移和氧电催化中的轨道相互作用。
氧气的释放和还原反应在决定水循环的效率(H 2 O⇔H 2 +Ø 2),其中氢用作能量载体。这就要求对氧电催化法有一个全面的了解,以进行有效的催化剂设计。关于氧电催化的当前观点集中在反应物/催化剂上的中间吸附的热力学上。因为氧分子是顺磁性的,所以其从反磁性氢氧化物/水中产生或还原成反磁性氢氧化物/水都涉及自旋相关的电子转移。催化剂与反应物/中间体之间的电子转移和轨道相互作用都显示出自旋依赖性,使反应动力学和热力学对自旋构型敏感。在此,简要介绍了自旋电子学,以解释氧释放反应(OER)和氧还原反应(ORR)的催化现象。以OER和ORR为基准的过渡金属基催化剂中的局部自旋构型和轨道相互作用为例进行了分析。为了进一步了解自旋电子氧电催化作用并开发出更有效的自旋电子催化剂,总结了挑战并提出了未来的机会。自旋电催化在不久的将来可能会成为一个重要的话题,并有助于整合对氧电催化的全面理解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号