Phytochemistry Letters ( IF 1.3 ) Pub Date : 2020-08-08 , DOI: 10.1016/j.phytol.2020.07.015 Pablo A. Chacón-Morales , Carolina Santiago-Dugarte , Juan M. Amaro-Luis
|
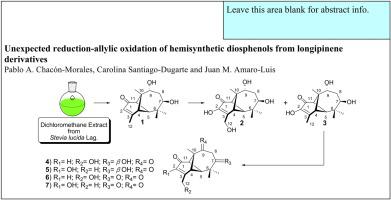
The 7β,9α-dihydroxylongipin-2-en-1-one (1) was obtained by basic hydrolysis of a dichloromethane extract of the resinous exudate from aerial parts of Stevia lucida. The treatment of 1 with potassium permanganate in concentrated hydrochloric acid generated two diosphenolic derivatives (2 and 3) one of which showed an unexpected allylic oxidation (2). Diosphenol 3 was treated with Jones reagent yielding four oxidation products (4-7). Two of these (4 and 6) have structural changes that suggest a process of reduction of the enolic double bond of diosphenol and another process of allylic oxidation. To explain the formation of the compounds 4 and 6 a plausible reaction mechanism based on a sequence of isomerizations was proposed. The structures of (1-7) were established based on NMR (1D and 2D) data interpretation.
中文翻译:

来自longipinene衍生物的半合成二酚的意外还原烯丙基氧化
7β,9α-dihydroxylongipin-2-en-1-one(1)是通过从甜叶菊地上部分的树脂渗出液的二氯甲烷提取物碱性水解得到的。用高锰酸钾在浓盐酸中处理1生成了两种二酚衍生物(2和3),其中之一显示出意想不到的烯丙基氧化(2)。Diosphenol 3用琼斯试剂,得到4个氧化产物(处理过的4 - 7)。其中两个(4和6)的结构变化表明一个还原二酚的烯丙基双键的过程和另一个烯丙基氧化的过程。为了解释化合物4和6的形成,提出了基于一系列异构化的合理的反应机理。的(结构1 - 7基于NMR(1D和2D)数据解释建立)。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号