当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Aspects of Phosphate Diester Cleavage Assisted by Imidazole. A Template Reaction for Obtaining Aryl Phosphoimidazoles
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-07-13 00:00:00 , DOI: 10.1021/acs.joc.6b01358 Mozart S. Pereira 1 , Bárbara Murta 1 , Thaís C. F. Oliveira 1 , Alex M. Manfredi 2 , Faruk Nome 2 , Alvan C. Hengge 3 , Tiago A. S. Brandão 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-07-13 00:00:00 , DOI: 10.1021/acs.joc.6b01358 Mozart S. Pereira 1 , Bárbara Murta 1 , Thaís C. F. Oliveira 1 , Alex M. Manfredi 2 , Faruk Nome 2 , Alvan C. Hengge 3 , Tiago A. S. Brandão 1
Affiliation
|
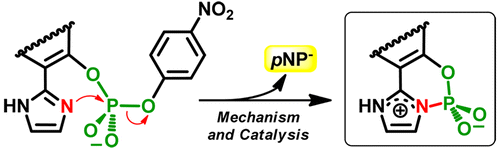
Phosphoimidazole-containing compounds are versatile players in biological and chemical processes. We explore catalytic and mechanistic criteria for the efficient formation of cyclic aryl phosphoimidazoles in aqueous solution, viewed as a template reaction for the in situ synthesis of related compounds. To provide a detailed analysis for this reaction a series of o-(2′-imidazolyl)naphthyl (4-nitrophenyl) phosphate isomers were examined to provide a basis for analysis of both mechanism and the influence of structural factors affecting the nucleophilic attack of the imidazolyl group on the phosphorus center of the substrate. Formation of the cyclic aryl phosphoimidazoles was probed by NMR and ESI-MS techniques. Kinetic experiments show that cyclization is faster under alkaline conditions, with an effective molarity up to 2900 M for the imidazolyl group, ruling out competition from external nucleophiles. Heavy atom isotope effect and computational studies show that the reaction occurs through a SN2(P)-type mechanism involving a pentacoordinated phosphorus TS, with apical positions occupied by the incoming imidazolyl nucleophile and the p-nitrophenolate leaving group. The P–O bond to the leaving group is about 50–60% broken in the transition state.
中文翻译:

咪唑辅助的磷酸二酯裂解的机械方面。获得芳基磷酸咪唑的模板反应
含磷咪唑的化合物是生物和化学过程中的多用途参与者。我们探索在水溶液中有效形成环状芳基磷酸咪唑的催化和机理标准,这被视为相关化合物的原位合成的模板反应。要用于该反应提供了详细的分析一系列ö研究了-(2'-咪唑基)萘基(4-硝基苯基)磷酸酯异构体,为分析影响咪唑基对底物磷中心的亲核进攻的机理和结构因素的影响提供了基础。通过NMR和ESI-MS技术探测环状芳基磷酸咪唑的形成。动力学实验表明,环化反应在碱性条件下较快,咪唑基的有效摩尔浓度高达2900 M,排除了与外部亲核试剂的竞争。重原子同位素效应和计算研究表明,该反应是通过S N 2(P)型机理发生的,该机理涉及五配位磷TS,其顶位被传入的咪唑基亲核体和p占据。-硝基酚盐离去基团。在过渡状态下,离去基团的P-O键断裂约50-60%。
更新日期:2016-07-13
中文翻译:

咪唑辅助的磷酸二酯裂解的机械方面。获得芳基磷酸咪唑的模板反应
含磷咪唑的化合物是生物和化学过程中的多用途参与者。我们探索在水溶液中有效形成环状芳基磷酸咪唑的催化和机理标准,这被视为相关化合物的原位合成的模板反应。要用于该反应提供了详细的分析一系列ö研究了-(2'-咪唑基)萘基(4-硝基苯基)磷酸酯异构体,为分析影响咪唑基对底物磷中心的亲核进攻的机理和结构因素的影响提供了基础。通过NMR和ESI-MS技术探测环状芳基磷酸咪唑的形成。动力学实验表明,环化反应在碱性条件下较快,咪唑基的有效摩尔浓度高达2900 M,排除了与外部亲核试剂的竞争。重原子同位素效应和计算研究表明,该反应是通过S N 2(P)型机理发生的,该机理涉及五配位磷TS,其顶位被传入的咪唑基亲核体和p占据。-硝基酚盐离去基团。在过渡状态下,离去基团的P-O键断裂约50-60%。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号