当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Prediction of the Potential Applications of Phenanthroline Derivatives in Separation of Transplutonium Elements.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.inorgchem.0c01271 Yang Liu 1 , Cong-Zhi Wang 1 , Qun-Yan Wu 1 , Jian-Hui Lan 1 , Zhi-Fang Chai 1, 2 , Qi Liu 3 , Wei-Qun Shi 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.inorgchem.0c01271 Yang Liu 1 , Cong-Zhi Wang 1 , Qun-Yan Wu 1 , Jian-Hui Lan 1 , Zhi-Fang Chai 1, 2 , Qi Liu 3 , Wei-Qun Shi 1
Affiliation
|
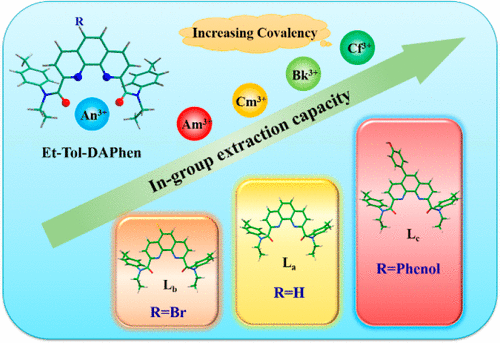
Recovery of transplutonium elements from adjacent actinides is extremely complicated in spent fuel reprocessing. Uncovering the electronic structures of transplutonium compounds is essential for designing robust ligands for in-group separation of transplutonium actinides. Here, we demonstrate the in-group transplutonium actinides separation ability of the recent developed phenanthroline ligand Et-Tol-DAPhen (N2,N9-diethyl-N2,N9-di-p-tolyl-1,10-phenanthroline-2,9-dicarboxamide, La) and its derivatives (5-bromo-(N2,N9-diethyl-N2,N9-di-p-tolyl-1,10-phenanthroline-2,9-dicarboxamide, Lb), and 5-(4-(λ1-oxidaneyl)phenyl)-(N2,N9-diethyl-N2,N9-di-p-tolyl-1,10-phenanthroline-2,9- dicarboxamide, Lc) through quasi-relativistic density functional theory (DFT). Both electrostatic potential and molecular orbital analyses of the ligands indicate that the electron-donating group substituted ligand Lc is a better electron donor to actinides than La and Lb. The possible extracted complexes AnL(NO3)3 and [AnL2(NO3)]2+ (L = La, Lb, Lc; An = Am, Cm, Bk, Cf) possess similar structures. Bonding nature analysis validates that the covalent interactions of the metal–ligand bonds are enhanced across actinide series from Am to Cf, which stem from the energy degeneracy of the 5f orbitals of actinides and the 2p orbitals of the ligand coordinating atoms. The Lc ligand displays slightly stronger covalent bonding compared to the other two ligands. Simultaneously, thermodynamic analysis confirms the stronger metal–ligand bonding of the Cf3+ complexes and the higher stability of the extraction species with Lc. Consequently, the covalency between the DAPhen derivatives and transplutonium actinides seems to be positively correlated with the extraction ability of these ligands. Nevertheless, these ligands exhibit diverse separation abilities to in-group actinide recovery. Therefore, the enhancement of covalency does not necessarily lead to the improvement of separation ability due to different extraction capabilities. We hope that these results will provide some inspiration for designing novel ligands for in-group transplutonium separation.
中文翻译:

菲咯啉衍生物在跨p元素分离中的潜在应用的理论预测。
在乏燃料后处理中,从相邻的act系元素中回收跨p元素非常复杂。揭露跨compounds化合物的电子结构对于设计用于跨in系元素组内分离的稳健配体至关重要。在这里,我们证明了组内transplutonium锕最近发达菲咯啉配位体的Et-TOL-DAPhen的分离能力(Ñ 2,Ñ 9二乙基Ñ 2,Ñ 9 -二p -甲苯基-1,10- phenanthroline- 2,9-二甲酰胺,L a)及其衍生物(5-溴-(N 2,N 9-二乙基-N 2,Ñ 9 -二p -甲苯基-1,10-菲咯啉-2,9-二甲酰胺,L b),和5-(4-(λ 1 -oxidaneyl)苯基) - (Ñ 2,Ñ 9二乙基ñ 2,ñ 9 -二p -甲苯基-1,10-菲咯啉,2,9-二甲酰胺,L C ^)通过准相对论密度泛函理论(DFT)。配位体的静电势和分子轨道分析均表明,供电子基团取代的配体L c比act a和L b是更好的to系电子供体。。可能提取的配合物AnL(NO 3)3和[AnL 2(NO 3)] 2+(L = L a,L b,L c; An = Am,Cm,Bk,Cf)具有相似的结构。键合性质分析证实,金属-配体键的共价相互作用在从Am到Cf的act系元素系列中得到增强,这是由于series系元素的5f轨道和配体配位原子的2p轨道的能量简并。与其他两个配体相比,L c配体显示出稍强的共价键。同时,热力学分析证实了Cf 3+的更强的金属-配体结合配合物和L c提取物的较高稳定性。因此,DAPhen衍生物与跨p系act素之间的共价似乎与这些配体的提取能力呈正相关。然而,这些配体表现出对组内act系元素回收的多种分离能力。因此,由于不同的提取能力,共价性的提高不一定导致分离能力的提高。我们希望这些结果将为设计用于组内跨p分离的新型配体提供一些启发。
更新日期:2020-08-17
中文翻译:

菲咯啉衍生物在跨p元素分离中的潜在应用的理论预测。
在乏燃料后处理中,从相邻的act系元素中回收跨p元素非常复杂。揭露跨compounds化合物的电子结构对于设计用于跨in系元素组内分离的稳健配体至关重要。在这里,我们证明了组内transplutonium锕最近发达菲咯啉配位体的Et-TOL-DAPhen的分离能力(Ñ 2,Ñ 9二乙基Ñ 2,Ñ 9 -二p -甲苯基-1,10- phenanthroline- 2,9-二甲酰胺,L a)及其衍生物(5-溴-(N 2,N 9-二乙基-N 2,Ñ 9 -二p -甲苯基-1,10-菲咯啉-2,9-二甲酰胺,L b),和5-(4-(λ 1 -oxidaneyl)苯基) - (Ñ 2,Ñ 9二乙基ñ 2,ñ 9 -二p -甲苯基-1,10-菲咯啉,2,9-二甲酰胺,L C ^)通过准相对论密度泛函理论(DFT)。配位体的静电势和分子轨道分析均表明,供电子基团取代的配体L c比act a和L b是更好的to系电子供体。。可能提取的配合物AnL(NO 3)3和[AnL 2(NO 3)] 2+(L = L a,L b,L c; An = Am,Cm,Bk,Cf)具有相似的结构。键合性质分析证实,金属-配体键的共价相互作用在从Am到Cf的act系元素系列中得到增强,这是由于series系元素的5f轨道和配体配位原子的2p轨道的能量简并。与其他两个配体相比,L c配体显示出稍强的共价键。同时,热力学分析证实了Cf 3+的更强的金属-配体结合配合物和L c提取物的较高稳定性。因此,DAPhen衍生物与跨p系act素之间的共价似乎与这些配体的提取能力呈正相关。然而,这些配体表现出对组内act系元素回收的多种分离能力。因此,由于不同的提取能力,共价性的提高不一定导致分离能力的提高。我们希望这些结果将为设计用于组内跨p分离的新型配体提供一些启发。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号