当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Role of Interfaces in Controlling Pb2+ Removal by Calcium Carbonate Minerals
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.cgd.0c00906
Fulvio Di Lorenzo 1 , Georgia Cametti 1 , Dimitri Vanhecke 2 , Sergey V. Churakov 1, 3
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.cgd.0c00906
Fulvio Di Lorenzo 1 , Georgia Cametti 1 , Dimitri Vanhecke 2 , Sergey V. Churakov 1, 3
Affiliation
|
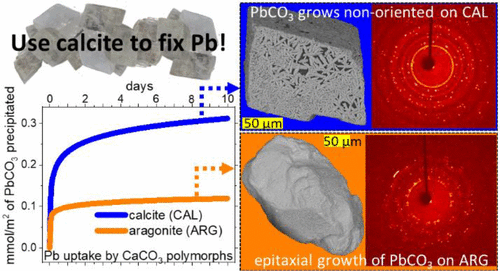
The possibility to develop a process for lead removal from wastewater, based on calcium carbonate minerals, depends on the overall efficiency of the uptake process. Aqueous Pb tends to form cerussite (CER) via a dissolution–precipitation reaction when interacting with aragonite (ARG) and calcite (CAL). From a thermodynamic perspective, the two processes have a similar driving force, because the solubility of CAL and ARG is approximatively the same (Ks ≈ 1 × 10–8). Experimentally, the macroscopic yield of reaction was found to be very different. Using ex situ electron microscopy, diffraction, and in situ atomic force microscopy, we demonstrate that the Pb uptake mechanism by the two most abundant CaCO3 polymorphs is controlled by the kinetics of processes at the solid–solid and solid–liquid interfaces. Aragonite is isostructural with the product phase (CER) that easily precipitates taking advantage of the template effect offered by the surfaces of the substrate. The reaction proceeds through an interface-coupled dissolution–precipitation that leads to a mineral replacement. Because of a crystallographic mismatch, the reaction between CAL and CER mainly occurs as a simple solvent-mediated transformation. Our study unveiled the mechanistic reasons behind the different reaction yields shown by CAL and ARG toward Pb uptake. Similar conclusions can be extended to other contaminant(aq)-CaCO3(s) systems, thus increasing the predictability of limestone efficiency toward the uptake of heavy metals.
中文翻译:

界面在控制碳酸钙矿物去除Pb 2+中的作用
开发基于碳酸钙矿物质从废水中去除铅的方法的可能性取决于吸收过程的整体效率。当与文石(ARG)和方解石(CAL)相互作用时,铅水溶液倾向于通过溶解-沉淀反应形成陶粒(CER)。从热力学的观点来看,这两个过程具有相似的驱动力,因为CAL和ARG的溶解度近似相同(ķ小号≈1×10 -8)。在实验上,发现反应的宏观产率非常不同。使用异位电子显微镜,衍射和原位原子力显微镜,我们证明了两种最丰富的CaCO 3吸收Pb的机理多晶型物受固体-固体和固体-液体界面的动力学控制。文石与产物相(CER)是同构的,可以利用基材表面提供的模板效应轻松沉淀。反应通过界面耦合的溶解-沉淀过程进行,导致矿物替代。由于晶体学上的不匹配,CAL和CER之间的反应主要以简单的溶剂介导的转化形式发生。我们的研究揭示了CAL和ARG对Pb吸收显示出不同的反应产率背后的机理原因。类似的结论可以推广到其他污染物(aq) -CaCO 3(s) 系统,从而提高了石灰石效率对重金属吸收的可预测性。
更新日期:2020-09-02
中文翻译:

界面在控制碳酸钙矿物去除Pb 2+中的作用
开发基于碳酸钙矿物质从废水中去除铅的方法的可能性取决于吸收过程的整体效率。当与文石(ARG)和方解石(CAL)相互作用时,铅水溶液倾向于通过溶解-沉淀反应形成陶粒(CER)。从热力学的观点来看,这两个过程具有相似的驱动力,因为CAL和ARG的溶解度近似相同(ķ小号≈1×10 -8)。在实验上,发现反应的宏观产率非常不同。使用异位电子显微镜,衍射和原位原子力显微镜,我们证明了两种最丰富的CaCO 3吸收Pb的机理多晶型物受固体-固体和固体-液体界面的动力学控制。文石与产物相(CER)是同构的,可以利用基材表面提供的模板效应轻松沉淀。反应通过界面耦合的溶解-沉淀过程进行,导致矿物替代。由于晶体学上的不匹配,CAL和CER之间的反应主要以简单的溶剂介导的转化形式发生。我们的研究揭示了CAL和ARG对Pb吸收显示出不同的反应产率背后的机理原因。类似的结论可以推广到其他污染物(aq) -CaCO 3(s) 系统,从而提高了石灰石效率对重金属吸收的可预测性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号