当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Organocatalytic Synthesis of δ‐Lactone‐Fused 4‐Chromanones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-04 , DOI: 10.1002/adsc.202000667
Yu‐Ting Lai, Koppanathi Nagaraju, Ramani Gurubrahamam, Kwunmin Chen
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-04 , DOI: 10.1002/adsc.202000667
Yu‐Ting Lai, Koppanathi Nagaraju, Ramani Gurubrahamam, Kwunmin Chen
|
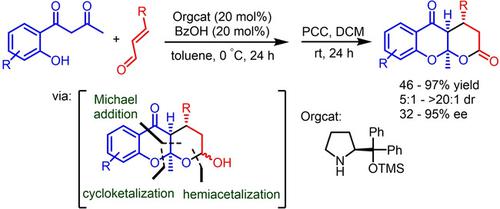
An organocatalytic enantioselective synthesis of δ‐lactone‐fused 4‐chromanones was demonstrated using 1‐(2‐hydroxyaryl)‐1,3‐butanedione and α,β‐unsaturated aldehydes in the presence of the Jørgensen‐Hayashi catalyst. The reaction proceeded through a Michael addition/cycloketalization/hemiacetalization sequence, and the resulting hemiacetals were oxidized into the corresponding lactone derivatives in a one‐pot manner. The desired fused O,O‐ketal tricyclic lactone motifs containing three contiguous chiral centers were obtained in excellent chemical yields (up to 97%) and with high stereoselectivities (up to >20:1 dr and up to 95% ee).
中文翻译:

对映体有机催化合成δ-内酯融合的4-色曼酮
在Jørgensen-Hayashi催化剂的存在下,使用1-(2-羟基芳基)-1,3-丁二酮和α,β-不饱和醛证明了δ-内酯融合的4-苯并二氢醌的有机催化对映选择性合成。反应按照迈克尔加成/环缩酮化/半缩醛化顺序进行,然后将生成的半缩醛以单锅方式氧化为相应的内酯衍生物。所需的包含三个连续手性中心的稠合的O,O-缩酮三环内酯基序以优异的化学收率(高达97%)和高的立体选择性(高达> 20:1 dr和高达95%ee)获得。
更新日期:2020-09-21
中文翻译:

对映体有机催化合成δ-内酯融合的4-色曼酮
在Jørgensen-Hayashi催化剂的存在下,使用1-(2-羟基芳基)-1,3-丁二酮和α,β-不饱和醛证明了δ-内酯融合的4-苯并二氢醌的有机催化对映选择性合成。反应按照迈克尔加成/环缩酮化/半缩醛化顺序进行,然后将生成的半缩醛以单锅方式氧化为相应的内酯衍生物。所需的包含三个连续手性中心的稠合的O,O-缩酮三环内酯基序以优异的化学收率(高达97%)和高的立体选择性(高达> 20:1 dr和高达95%ee)获得。































 京公网安备 11010802027423号
京公网安备 11010802027423号