当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dietary Polyphenols Inhibit the Cytochrome P450 Monooxygenase Branch of the Arachidonic Acid Cascade with Remarkable Structure-Dependent Selectivity and Potency.
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-08-03 , DOI: 10.1021/acs.jafc.0c04690 Nadja Kampschulte 1 , Ayah Alasmer 1 , Michael T Empl 2 , Michael Krohn 1 , Pablo Steinberg 2 , Nils Helge Schebb 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-08-03 , DOI: 10.1021/acs.jafc.0c04690 Nadja Kampschulte 1 , Ayah Alasmer 1 , Michael T Empl 2 , Michael Krohn 1 , Pablo Steinberg 2 , Nils Helge Schebb 1, 2
Affiliation
|
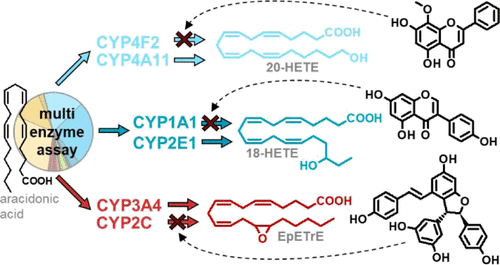
The products of the cytochrome P450 monooxygenase (CYP)-catalyzed oxidation of arachidonic acid (AA), that is, epoxy- and hydroxy-fatty acids, play a crucial role in the homeostasis of several physiological processes. In a liver microsome-based multienzyme assay using AA as natural substrate, we investigated how polyphenols inhibit different oxylipin-forming CYP in parallel but independently from each other. The ω-hydroxylating CYP4F2 and CYP4A11 were investigated, as well as the epoxidizing CYP2C-subfamily and CYP3A4 along with the (ω-n)-hydroxylating CYP1A1 and CYP2E1. The oxylipin formation was inhibited by several polyphenols with a remarkable selectivity and a potency comparable to known CYP inhibitors. The flavone apigenin inhibited the epoxidation, ω-hydroxylation, and (ω-n)-hydroxylation of AA with IC50 values of 4.4–9.8, 2.9–10, and 10–25 μM, respectively. Other flavones such as wogonin selectively inhibited CYP1A1-catalyzed (ω-n)-hydroxylation with an IC50 value of 0.10–0.22 μM, while the isoflavone genistein was a selective ω-hydroxylase inhibitor (IC50: 5.5–46 μM). Of note, the flavanone naringenin and the anthocyanidin perlargonidin did not inhibit CYPs of the AA cascade. Moderate permeability of apigenin as tested in the Caco-2 model of intestinal absorption (Papp: 4.5 ± 1 × 10–6 cm/s) and confirmation of the inhibition of 20-HETE formation by apigenin in the colorectal cancer-derived cell line HCT 116 (IC50: 1.5–8.8 μM) underline the possible in vivo relevance of these effects. Further research is needed to better understand how polyphenols impact human health by this newly described molecular mode of action.
中文翻译:

饮食中的多酚具有明显的结构依赖性选择性和效力,可抑制花生四烯酸级联的细胞色素P450单加氧酶分支。
细胞色素P450单加氧酶(CYP)催化花生四烯酸(AA)氧化的产物,即环氧和羟基脂肪酸,在几种生理过程的体内平衡中起关键作用。在使用AA作为天然底物的基于肝微粒体的多酶测定中,我们研究了多酚如何平行但彼此独立地抑制不同的形成脂蛋白的CYP。研究了ω-羟基化CYP4F2和CYP4A11,以及环氧化CYP2C亚家族和CYP3A4,以及(ω- n)-羟基化CYP1A1和CYP2E1。与几种已知的CYP抑制剂相比,几种多酚可抑制oxylipin的形成,其选择性和效价极高。黄酮芹菜素抑制环氧化,ω-羟基化和(ω- n)-AA羟基化,IC 50值分别为4.4–9.8、2.9–10和10–25μM。其他黄酮类化合物,如沃戈宁选择性抑制CYP1A1催化的(ω- n)-羟基化,IC 50值为0.10-0.22μM,而异黄酮染料木黄酮为选择性ω-羟化酶抑制剂(IC 50:5.5-46μM)。值得一提的是,黄烷酮柚皮苷和花青素过氧化物酶没有抑制AA级联的CYP。如在肠道吸收的Caco-2模型中测试的芹菜素适度通透性(P app:4.5±1×10 –6 cm / s),并证实了芹菜素在结直肠癌衍生的细胞系中抑制20-HETE形成HCT 116(IC 50:1.5–8.8μM)强调了这些效应可能与体内相关。需要进一步的研究,以更好地了解多酚如何通过这种新描述的分子作用方式影响人类健康。
更新日期:2020-08-26
中文翻译:

饮食中的多酚具有明显的结构依赖性选择性和效力,可抑制花生四烯酸级联的细胞色素P450单加氧酶分支。
细胞色素P450单加氧酶(CYP)催化花生四烯酸(AA)氧化的产物,即环氧和羟基脂肪酸,在几种生理过程的体内平衡中起关键作用。在使用AA作为天然底物的基于肝微粒体的多酶测定中,我们研究了多酚如何平行但彼此独立地抑制不同的形成脂蛋白的CYP。研究了ω-羟基化CYP4F2和CYP4A11,以及环氧化CYP2C亚家族和CYP3A4,以及(ω- n)-羟基化CYP1A1和CYP2E1。与几种已知的CYP抑制剂相比,几种多酚可抑制oxylipin的形成,其选择性和效价极高。黄酮芹菜素抑制环氧化,ω-羟基化和(ω- n)-AA羟基化,IC 50值分别为4.4–9.8、2.9–10和10–25μM。其他黄酮类化合物,如沃戈宁选择性抑制CYP1A1催化的(ω- n)-羟基化,IC 50值为0.10-0.22μM,而异黄酮染料木黄酮为选择性ω-羟化酶抑制剂(IC 50:5.5-46μM)。值得一提的是,黄烷酮柚皮苷和花青素过氧化物酶没有抑制AA级联的CYP。如在肠道吸收的Caco-2模型中测试的芹菜素适度通透性(P app:4.5±1×10 –6 cm / s),并证实了芹菜素在结直肠癌衍生的细胞系中抑制20-HETE形成HCT 116(IC 50:1.5–8.8μM)强调了这些效应可能与体内相关。需要进一步的研究,以更好地了解多酚如何通过这种新描述的分子作用方式影响人类健康。































 京公网安备 11010802027423号
京公网安备 11010802027423号