当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Propyne Hydrogenation over a Pd/Cu(111) Single-Atom Alloy Studied using Ambient Pressure Infrared Spectroscopy
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-08-03 , DOI: 10.1021/acscatal.0c02475 Mohammed K. Abdel-Rahman 1 , Michael Trenary 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-08-03 , DOI: 10.1021/acscatal.0c02475 Mohammed K. Abdel-Rahman 1 , Michael Trenary 1
Affiliation
|
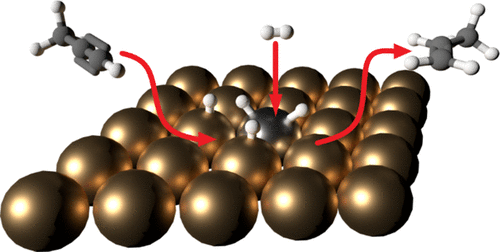
The hydrogenation of propyne (C3H4) to propene (C3H6) using a Pd/Cu(111) single-atom alloy (SAA) has been studied using polarization-dependent reflection absorption infrared spectroscopy. This method allows for simultaneous monitoring of reactants and products in the gas phase and species adsorbed on the surface during the reaction. The results were compared with the hydrogenation of propyne using Pd-free Cu(111) as well as with previous studies on Pd/Cu SAA catalysts supported on alumina. Propene production occurs at temperatures of 383 K and above as indicated by the appearance of an infrared peak at 912 cm–1, which is a unique characteristic feature of gas phase propene. Propyne was found to adsorb on the surface at 300 K in the presence of gas phase propyne to form a di-σ/di-π structure, as the spectrum was identical to that reported in the literature for propyne adsorbed on Cu(111) at 150 K in ultrahigh vacuum. The presence of a carbonaceous layer on the surface is indicated by a dramatic increase in the intensity of a peak at 2968 cm–1 at temperatures above 400 K. The progression of gas phase peaks at 912 and 3322 cm–1 was used to calculate the rate of production of propene and the rate of consumption of propyne, respectively. This reaction rate was used to determine a turnover frequency of 25.4 s–1 at 383 K for the reaction on the Pd/Cu(111) SAA surface. The reaction was not impeded by the presence of the carbonaceous layer, even for a layer so thick that only carbon was detectable on the surface with Auger electron spectroscopy.
中文翻译:

使用常压红外光谱研究Pd / Cu(111)单原子合金上的丙炔氢化
使用偏振相关的反射吸收红外光谱研究了使用Pd / Cu(111)单原子合金(SAA)将丙炔(C 3 H 4)加氢成丙烯(C 3 H 6)。该方法允许同时监测气相中的反应物和产物以及反应期间吸附在表面上的物质。将结果与使用无Pd的Cu(111)进行丙炔的氢化以及以前对氧化铝上负载的Pd / Cu SAA催化剂的研究进行了比较。丙烯的产生发生在383 K和更高的温度下,如在912 cm –1处出现红外峰所示,这是气相丙烯的独特特征。发现丙炔在气相丙炔存在下在300 K时吸附在表面上,形成di-σ/di-π结构,因为其光谱与文献报道的丙炔在Cu(111)上吸附的光谱相同。 150 K超高真空。当温度高于400 K时,在2968 cm –1处的峰强度显着增加,表明表面存在碳质层。气相色谱峰在912和3322 cm –1处的变化用来计算碳的含量。丙烯的生产率和丙炔的消耗率。该反应速率用于确定25.4 s –1的周转频率Pd / Cu(111)SAA表面上的反应温度为383K。碳层的存在并不会阻碍反应,即使对于如此厚的层,通过俄歇电子能谱也只能在表面检测到碳。
更新日期:2020-09-05
中文翻译:

使用常压红外光谱研究Pd / Cu(111)单原子合金上的丙炔氢化
使用偏振相关的反射吸收红外光谱研究了使用Pd / Cu(111)单原子合金(SAA)将丙炔(C 3 H 4)加氢成丙烯(C 3 H 6)。该方法允许同时监测气相中的反应物和产物以及反应期间吸附在表面上的物质。将结果与使用无Pd的Cu(111)进行丙炔的氢化以及以前对氧化铝上负载的Pd / Cu SAA催化剂的研究进行了比较。丙烯的产生发生在383 K和更高的温度下,如在912 cm –1处出现红外峰所示,这是气相丙烯的独特特征。发现丙炔在气相丙炔存在下在300 K时吸附在表面上,形成di-σ/di-π结构,因为其光谱与文献报道的丙炔在Cu(111)上吸附的光谱相同。 150 K超高真空。当温度高于400 K时,在2968 cm –1处的峰强度显着增加,表明表面存在碳质层。气相色谱峰在912和3322 cm –1处的变化用来计算碳的含量。丙烯的生产率和丙炔的消耗率。该反应速率用于确定25.4 s –1的周转频率Pd / Cu(111)SAA表面上的反应温度为383K。碳层的存在并不会阻碍反应,即使对于如此厚的层,通过俄歇电子能谱也只能在表面检测到碳。































 京公网安备 11010802027423号
京公网安备 11010802027423号