Biomedicine & Pharmacotherapy ( IF 6.9 ) Pub Date : 2020-08-04 , DOI: 10.1016/j.biopha.2020.110556 Zhe Wu 1 , Yaqiong Zhan 1 , Li Wang 1 , Jiepeng Tong 1 , Li Zhang 1 , Mengjia Lin 1 , Xuehang Jin 1 , Lushun Jiang 1 , Yan Lou 1 , Yunqing Qiu 1
|
Backgrounds
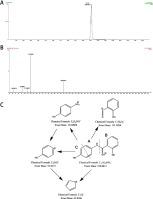
Ribonucleotide reductase (RR) catalyzes the essential step in the formation of all four deoxynucleotides. Upregulated activity of RR plays an active role in tumor progression. As the regulatory subunit of RR, ribonucleotide reductase subunit M2 (RRM2) is regarded as one of the effective therapeutic targets for DNA replication-dependent diseases, such as cancers. Recent studies have revealed that osalmid significantly inhibits the activity of RRM2, but the metabolic profile of osalmid remains unknown.
Objective
The aim of this study was to clarify the metabolic profile including metabolites, isoenzymes and metabolic pathways of osalmid. The anti-human hepatocellular carcinoma activity and mechanism of metabolites were further investigated.
Materials and methods
Ultra high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS) was used for identifying metabolites and for characterizing phase I and phase II metabolic pathways with recombinant enzymes or in human liver microsomes of osalmid. The eHiTS docking system was used for potential RRM2 inhibitor screening among metabolites. Cytotoxicity assays were performed for evaluating cell proliferation inhibitory activity of metabolites. Cell cycle assays and cell apoptosis assays were assessed by flow cytometry. Western blotting analysis of RRM2, cyclin D1, p21, p53, phosphorylated p53, Bcl-2 and Bax was performed to explore the anti-hepatocellular carcinoma mechanism of the active metabolites.
Results
Ten metabolites of osalmid were identified, and none of them have been reported previously. Hydroxylation, glucuronidation, sulfonation, acetylation and degradation were recognized as the main metabolic processes of osalmid. Isozymes of CYP1A2, CYP2C9, UGT1A1, UGT1A6, UGT1A9, UGT2B7 and UGT2B15 were involved in phase I and phase II metabolism of osalmid. Metabolites M7, M8 and M10 showed higher binding affinities with the RRM2 active site than osalmid. Metabolite M7 exhibited potent inhibitory activity to hepatocellular carcinoma cell lines by both competitive inhibition and down-regulation of RRM2. Moreover, M7 significantly induced cell cycle arrest and apoptosis by activating p53-related pathways.
Conclusions
The metabolic profile of osalmid was identified. M7 significantly inhibited human hepatocellular carcinoma progression by inhibiting RRM2 activity. Furthermore, M7 induced cell cycle arrest and apoptosis by activating p53-related signaling pathways.
中文翻译:

鉴定人肝癌细胞中的osalmid代谢谱和具有抗肿瘤活性的活性代谢物。
背景资料
核糖核苷酸还原酶(RR)催化所有四个脱氧核苷酸形成的关键步骤。RR的上调活性在肿瘤进展中起积极作用。作为RR的调节亚基,核糖核苷酸还原酶亚基M2(RRM2)被认为是依赖DNA复制的疾病例如癌症的有效治疗靶标之一。最近的研究表明,osalmid显着抑制RRM2的活性,但是osalmid的代谢特征仍然未知。
目的
这项研究的目的是阐明代谢特征,包括代谢产物,同工酶和of的代谢途径。进一步研究了抗人肝癌的活性及其代谢产物的机制。
材料和方法
超高效液相色谱-四极杆飞行时间质谱( UPLC / Q-TOF-MS)用于鉴定代谢产物并表征重组酶在人肝微粒体中的Osalmid的I和II期代谢途径。eHiTS对接系统用于在代谢物中筛选潜在的RRM2抑制剂。进行细胞毒性测定以评估代谢产物的细胞增殖抑制活性。通过流式细胞术评估细胞周期测定法和细胞凋亡测定法。对RRM2,细胞周期蛋白D1,p21,p53,磷酸化的p53,Bcl-2和Bax进行了蛋白质印迹分析,以探索活性代谢物的抗肝癌机制。
结果
鉴定出10种osalmid代谢物,但以前均未报道。羟基化,葡萄糖醛酸化,磺化,乙酰化和降解被认为是杂的主要代谢过程。CYP1A2,CYP2C9,UGT1A1,UGT1A6,UGT1A9,UGT2B7和UGT2B15的同工酶参与的I和II期代谢。代谢产物M7,M8和M10与RRM2活性位点的结合亲和力高于osalmid。代谢物M7通过竞争抑制和下调RRM2表现出对肝癌细胞系的有效抑制活性。此外,M7通过激活p53相关途径显着诱导细胞周期停滞和凋亡。
结论
确定了osalmid的代谢特征。M7通过抑制RRM2活性来显着抑制人肝癌的进展。此外,M7通过激活p53相关信号通路诱导细胞周期停滞和凋亡。






























 京公网安备 11010802027423号
京公网安备 11010802027423号