当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed three-component carbonylative synthesis of 2-(trifluoromethyl)quinazolin-4(3H)-ones from trifluoroacetimidoyl chlorides and amines
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-03 , DOI: 10.1039/d0qo00819b Zhengkai Chen 1, 2, 3, 4 , Le-Cheng Wang 1, 2, 3, 4 , Jiajun Zhang 1, 2, 3, 4 , Xiao-Feng Wu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-03 , DOI: 10.1039/d0qo00819b Zhengkai Chen 1, 2, 3, 4 , Le-Cheng Wang 1, 2, 3, 4 , Jiajun Zhang 1, 2, 3, 4 , Xiao-Feng Wu 1, 2, 3, 4, 5
Affiliation
|
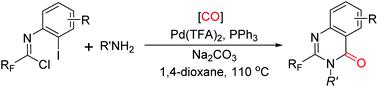
A palladium-catalyzed three-component carbonylative reaction of trifluoroacetimidoyl chlorides and amines for the synthesis of valuable 2-(trifluoromethyl)quinazolin-4(3H)-ones has been developed. Broad substrate scope (41 examples), high efficiency (up to 99% yield) and no manipulation of CO gas constitute the notable features of this reaction. The present strategy can be applied to the late-stage modification of natural molecules and to the synthesis of the bioactive alkaloid molecule rutaecarpine.
中文翻译:

由三氟乙酰亚胺基氯化物和胺催化钯催化的三组分羰基合成2-(三氟甲基)喹唑啉-4(3H)-
已经开发了钯催化的三氟乙酰亚胺基氯和胺的三组分羰基化反应,以合成有价值的2-(三氟甲基)喹唑啉-4(3 H)-一。广泛的底物范围(41个实例),高效率(高达99%的收率)和无需操作CO气体构成了该反应的显着特征。本发明的策略可以应用于天然分子的后期修饰和生物活性生物碱分子芸香芸香碱的合成。
更新日期:2020-08-25
中文翻译:

由三氟乙酰亚胺基氯化物和胺催化钯催化的三组分羰基合成2-(三氟甲基)喹唑啉-4(3H)-
已经开发了钯催化的三氟乙酰亚胺基氯和胺的三组分羰基化反应,以合成有价值的2-(三氟甲基)喹唑啉-4(3 H)-一。广泛的底物范围(41个实例),高效率(高达99%的收率)和无需操作CO气体构成了该反应的显着特征。本发明的策略可以应用于天然分子的后期修饰和生物活性生物碱分子芸香芸香碱的合成。








































 京公网安备 11010802027423号
京公网安备 11010802027423号