Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of the Novel AT1 Receptor Tracer [18F]Fluoropyridine-Candesartan via Click Chemistry.
ACS Omega ( IF 3.7 ) Pub Date : 2020-08-03 , DOI: 10.1021/acsomega.0c02310 Aida M Abreu Diaz 1, 2, 3, 4 , Gergana O Drumeva 1, 2 , Daniil R Petrenyov 1 , Jean-François Carrier 1, 3, 5, 6 , Jean N DaSilva 1, 2, 3, 6
ACS Omega ( IF 3.7 ) Pub Date : 2020-08-03 , DOI: 10.1021/acsomega.0c02310 Aida M Abreu Diaz 1, 2, 3, 4 , Gergana O Drumeva 1, 2 , Daniil R Petrenyov 1 , Jean-François Carrier 1, 3, 5, 6 , Jean N DaSilva 1, 2, 3, 6
Affiliation
|
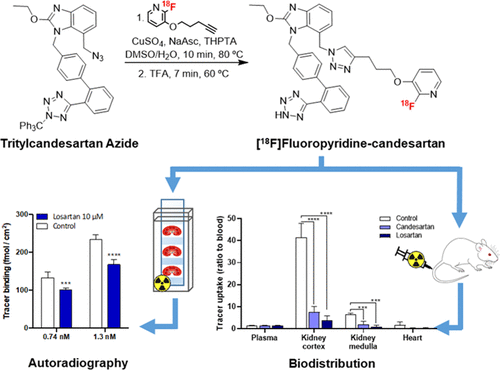
A novel 7-((4-(3-((2-[18F]fluoropyridin-3-yl)oxy)propyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole derivative of the angiotensin II type-1 receptor (AT1R) blocker candesartan, [18F]fluoropyridine–candesartan, was synthesized via the copper-catalyzed azide–alkyne cycloaddition click reaction between 2-[18F]fluoro-3-(pent-4-yn-1-yloxy)pyridine ([18F]FPyKYNE) and the tetrazole-protected azido-candesartan derivative, followed by acid deprotection. This three-step, two-pot, and two-step purification synthesis was done within 2 h. The use of tris[(1-hydroxypropyl-1H-1,2,3-triazol-4-yl)methyl]amine (THPTA) as a Cu(I) stabilizing agent increased the overall radiochemical yield by 4-fold (10 ± 2%, n = 13) compared to the reaction without THPTA (2.4 ± 0.2%, n = 3; decay-corrected from 18F produced at the end-of-beam). Complete separation of [18F]FPyKYNE from its nitro precursor and [18F]fluoropyridine–candesartan from the deprotected azido-candesartan allowed for high molar activities (>380 GBq/μmol) of the tracer. The use of 0.1% trifluoroacetic acid in water for reformulation and the addition of sodium ascorbate to the final formulation (1.6 ± 0.2 GBq/mL, n = 3) prevented tracer radiolysis with >97% radiochemical purity for a period of up to 10 h after the end-of-synthesis. A significant reduction in the uptake (86 ± 3%, n = 8) of the tracer was observed ex vivo in rats (at 20 min postinjection) in the AT1R-rich kidney cortex following pretreatment with saturating doses of the AT1R antagonist candesartan or losartan. This specific binding to AT1R was confirmed in vitro in the rat renal cortex (autoradiography) by a reduction of 26 ± 5% (n = 12) with losartan coincubation (10 μM). These favorable binding properties support further studies to assess the potential of [18F]fluoropyridine–candesartan as a tracer for the positron emission tomography imaging of renal AT1R.
中文翻译:

通过点击化学法合成新型AT1受体示踪剂[18F]氟吡啶-坎地沙坦。
新型7-((4-(3-((2- [ 18 -F]氟吡啶-3-基)氧基)丙基)-1 H -1,2,3-三唑-1-基)甲基)-1 H血管紧张素II 1型受体(AT 1 R)阻断剂坎地沙坦的[ -苯并[ d ]咪唑衍生物] [ 18 F]氟吡啶-坎地沙坦是通过铜催化的叠氮化物-炔烃环加成反应在2- [ 18 F ]氟-3-(戊-4-yn-1-基氧基)吡啶([ 18 F] FPyKYNE)和四唑保护的叠氮基-坎地沙坦衍生物,然后进行酸脱保护。此三步,两锅法和两步纯化合成在2小时内完成。使用三[[(1-羟丙基-1 H-1,2,3-三唑-4(基)甲基]胺(THPTA)作为铜(I)稳定剂,与反应相比,其总放射化学收率提高了4倍(10±2%,n = 13)没有THPTA(2.4±0.2%,n = 3;从光束末端产生的18 F进行衰减校正)。[完全分离18 F] FPyKYNE从其硝基前体和[ 18 F]从脱保护的叠氮基坎地允许高摩尔活动氟吡啶坎地沙坦(> 380吉贝/微摩尔)的示踪剂。在水中使用0.1%三氟乙酸进行配方调整,并向最终配方中添加抗坏血酸钠(1.6±0.2 GBq / mL,n= 3)在合成结束后长达10小时内,放射化学纯度> 97%的示踪剂放射裂解被阻止。在摄取甲显著减少(86±3%,Ñ观察到示踪物= 8)的离体大鼠(在20分钟注射后)在AT 1个富R肾皮质以下具有饱和剂量的AT预处理1 - [R拮抗剂坎地沙坦或氯沙坦。通过与氯沙坦共孵育(10μM)将大鼠肾皮质(放射自显影)中的AT 1 R特异性结合降低了26±5%(n = 12),从而证实了这种特异性结合。这些有利的结合特性为进一步研究评估[ 18F]氟吡啶-坎地沙坦作为肾脏AT 1 R正电子发射断层显像的示踪剂。
更新日期:2020-08-18
中文翻译:

通过点击化学法合成新型AT1受体示踪剂[18F]氟吡啶-坎地沙坦。
新型7-((4-(3-((2- [ 18 -F]氟吡啶-3-基)氧基)丙基)-1 H -1,2,3-三唑-1-基)甲基)-1 H血管紧张素II 1型受体(AT 1 R)阻断剂坎地沙坦的[ -苯并[ d ]咪唑衍生物] [ 18 F]氟吡啶-坎地沙坦是通过铜催化的叠氮化物-炔烃环加成反应在2- [ 18 F ]氟-3-(戊-4-yn-1-基氧基)吡啶([ 18 F] FPyKYNE)和四唑保护的叠氮基-坎地沙坦衍生物,然后进行酸脱保护。此三步,两锅法和两步纯化合成在2小时内完成。使用三[[(1-羟丙基-1 H-1,2,3-三唑-4(基)甲基]胺(THPTA)作为铜(I)稳定剂,与反应相比,其总放射化学收率提高了4倍(10±2%,n = 13)没有THPTA(2.4±0.2%,n = 3;从光束末端产生的18 F进行衰减校正)。[完全分离18 F] FPyKYNE从其硝基前体和[ 18 F]从脱保护的叠氮基坎地允许高摩尔活动氟吡啶坎地沙坦(> 380吉贝/微摩尔)的示踪剂。在水中使用0.1%三氟乙酸进行配方调整,并向最终配方中添加抗坏血酸钠(1.6±0.2 GBq / mL,n= 3)在合成结束后长达10小时内,放射化学纯度> 97%的示踪剂放射裂解被阻止。在摄取甲显著减少(86±3%,Ñ观察到示踪物= 8)的离体大鼠(在20分钟注射后)在AT 1个富R肾皮质以下具有饱和剂量的AT预处理1 - [R拮抗剂坎地沙坦或氯沙坦。通过与氯沙坦共孵育(10μM)将大鼠肾皮质(放射自显影)中的AT 1 R特异性结合降低了26±5%(n = 12),从而证实了这种特异性结合。这些有利的结合特性为进一步研究评估[ 18F]氟吡啶-坎地沙坦作为肾脏AT 1 R正电子发射断层显像的示踪剂。















































 京公网安备 11010802027423号
京公网安备 11010802027423号