Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Methyl-ligated tin silsesquioxane catalyzed reactions of glucose
Journal of Catalysis ( IF 6.5 ) Pub Date : 2016-07-14 05:03:50
Stephen K. Brand, Tyler R. Josephson, Jay A. Labinger, Stavros Caratzoulas, Dionisios G. Vlachos, Mark E. Davis
Journal of Catalysis ( IF 6.5 ) Pub Date : 2016-07-14 05:03:50
Stephen K. Brand, Tyler R. Josephson, Jay A. Labinger, Stavros Caratzoulas, Dionisios G. Vlachos, Mark E. Davis
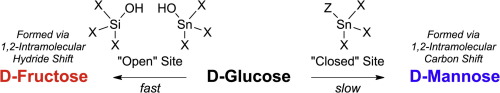
|
Tin-containing zeolite Beta (Sn-Beta) has been investigated as a catalyst for isomerizing aldohexoses into ketohexoses through a Lewis acid mediated hydride shift. Recent studies on the reactivities of Lewis base-doped and alkali-exchanged Sn-Beta samples have conclusively demonstrated that the “open” tin site performs the glucose isomerization reaction. With Lewis base doped Sn-Beta, glucose conversion is almost completely eliminated and product selectivity is shifted predominantly to mannose. These data suggest that glucose reactions may occur through pathways that do not involve the “open” site in Sn-Beta; albeit at significantly lower rates. To examine this possibility, reactions of glucose catalyzed by a homogeneous model of Sn-Beta that does not contain “open” sites, methyl-ligated tin silsesquioxane 1a, is experimentally and theoretically examined. 1a is an active glucose conversion catalyst selectively producing mannose, although the rates of reaction are far below those obtained from Sn-Beta. A hybrid quantum mechanical/molecular mechanics model is constructed, and the complete catalytic cycle is computationally examined, considering ring-opening, three distinct pathways for each hydride- and carbon-shift reaction, and ring-closing. The combined experimental and computational results suggest that there could be reaction pathways that involve Si–O–Sn cleavage that give much slower reaction rates than the open tin site in Sn-Beta.
中文翻译:

甲基连接的锡倍半硅氧烷锡催化葡萄糖反应
已经研究了含锡的β沸石(Sn-Beta)作为通过路易斯酸介导的氢化物转移将醛己糖异构化为酮己糖的催化剂。对路易斯碱掺杂和碱交换的Sn-Beta样品的反应性的最新研究已最终证明,“开放”锡位点可进行葡萄糖异构化反应。使用路易斯碱掺杂的Sn-β,几乎完全消除了葡萄糖转化,并且产物选择性主要转移到了甘露糖上。这些数据表明,葡萄糖反应可能通过不涉及Sn-Beta中“开放”位点的途径发生。尽管比率要低得多。为了检验这种可能性,可以使用不包含“开放”位点的均一的Sn-β均相模型催化的葡萄糖反应,即甲基连接的倍半硅氧烷锡1a,在实验和理论上进行了检查。尽管反应速率远低于从Sn-Beta获得的速率,但是图1a是选择性产生甘露糖的活性葡萄糖转化催化剂。建立了混合的量子力学/分子力学模型,并考虑了开环,每个氢化物和碳转移反应的三个不同途径以及闭环,对整个催化循环进行了计算检验。综合实验和计算结果表明,可能存在涉及Si–O–Sn裂解的反应途径,其反应速率比Sn-Beta中开放的锡位点慢得多。建立了混合的量子力学/分子力学模型,并考虑了开环,每个氢化物和碳转移反应的三个不同途径以及闭环,对整个催化循环进行了计算检验。综合实验和计算结果表明,可能存在涉及Si–O–Sn裂解的反应途径,其反应速率比Sn-Beta中开放的锡位点慢得多。建立了混合的量子力学/分子力学模型,并考虑了开环,每个氢化物和碳转移反应的三个不同途径以及闭环,对整个催化循环进行了计算检验。综合实验和计算结果表明,可能存在涉及Si–O–Sn裂解的反应途径,其反应速率比Sn-Beta中开放的锡位点慢得多。
更新日期:2016-07-14
中文翻译:

甲基连接的锡倍半硅氧烷锡催化葡萄糖反应
已经研究了含锡的β沸石(Sn-Beta)作为通过路易斯酸介导的氢化物转移将醛己糖异构化为酮己糖的催化剂。对路易斯碱掺杂和碱交换的Sn-Beta样品的反应性的最新研究已最终证明,“开放”锡位点可进行葡萄糖异构化反应。使用路易斯碱掺杂的Sn-β,几乎完全消除了葡萄糖转化,并且产物选择性主要转移到了甘露糖上。这些数据表明,葡萄糖反应可能通过不涉及Sn-Beta中“开放”位点的途径发生。尽管比率要低得多。为了检验这种可能性,可以使用不包含“开放”位点的均一的Sn-β均相模型催化的葡萄糖反应,即甲基连接的倍半硅氧烷锡1a,在实验和理论上进行了检查。尽管反应速率远低于从Sn-Beta获得的速率,但是图1a是选择性产生甘露糖的活性葡萄糖转化催化剂。建立了混合的量子力学/分子力学模型,并考虑了开环,每个氢化物和碳转移反应的三个不同途径以及闭环,对整个催化循环进行了计算检验。综合实验和计算结果表明,可能存在涉及Si–O–Sn裂解的反应途径,其反应速率比Sn-Beta中开放的锡位点慢得多。建立了混合的量子力学/分子力学模型,并考虑了开环,每个氢化物和碳转移反应的三个不同途径以及闭环,对整个催化循环进行了计算检验。综合实验和计算结果表明,可能存在涉及Si–O–Sn裂解的反应途径,其反应速率比Sn-Beta中开放的锡位点慢得多。建立了混合的量子力学/分子力学模型,并考虑了开环,每个氢化物和碳转移反应的三个不同途径以及闭环,对整个催化循环进行了计算检验。综合实验和计算结果表明,可能存在涉及Si–O–Sn裂解的反应途径,其反应速率比Sn-Beta中开放的锡位点慢得多。

































 京公网安备 11010802027423号
京公网安备 11010802027423号