当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bimetallic PtFe-Catalyzed Selective Hydrogenation of Furfural to Furfuryl Alcohol: Solvent Effect of Isopropanol and Hydrogen Activation
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-07-31 , DOI: 10.1021/acssuschemeng.0c04891 Xing Gao 1 , Suyang Tian 1 , Yunyun Jin 1 , Xiaoyue Wan 1 , Chunmei Zhou 1 , Rizhi Chen 2 , Yihu Dai 1 , Yanhui Yang 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-07-31 , DOI: 10.1021/acssuschemeng.0c04891 Xing Gao 1 , Suyang Tian 1 , Yunyun Jin 1 , Xiaoyue Wan 1 , Chunmei Zhou 1 , Rizhi Chen 2 , Yihu Dai 1 , Yanhui Yang 1
Affiliation
|
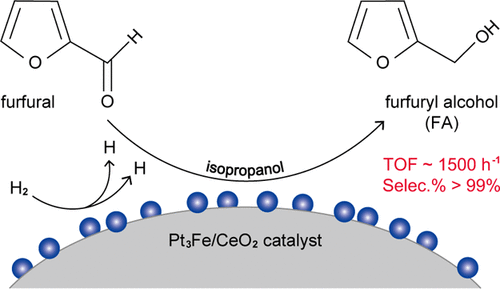
Highly selective liquid-phase hydrogenation of furfural was efficiently catalyzed by PtFe alloy nanoparticles supported on CeO2. An initial reaction rate at ∼1500 h–1, a near 100% selectivity toward furfuryl alcohol (FA), and a satisfactory recycling stability were achieved on Pt3Fe/CeO2 catalyst. Based on the well-established knowledge of the modification effects of Fe additives on the geometric and electronic properties of Pt sites, this work further demonstrated that the bimetallic PtFe sites could promote H2-activated dissociation for accelerating the hydrogenation reaction in comparison with the monometallic Pt/CeO2 catalyst. The results of isotopic and kinetic experiments to study the solvent effects elucidated that the reaction pathway of catalytic transfer hydrogenation with isopropanol as the hydrogen donor could be ruled out. The results also suggested that isopropanol-involved hydrogen exchange processes with hydrogen and FA could easily occur during the reactions. Furthermore, the furfural hydrogenation could be significantly affected by the solvent intermolecular hydrogen bonding interactions.
中文翻译:

PtFe双金属催化糠醛选择性氢化为糠醇:异丙醇的溶剂作用和氢活化
负载在CeO 2上的PtFe合金纳米粒子有效地催化了糠醛的高选择性液相加氢。在Pt 3 Fe / CeO 2催化剂上,初始反应速率约为1500 h –1,对糠醇(FA)的选择性接近100%,并且循环稳定性令人满意。基于对铁添加剂对Pt位的几何和电子性质的改性作用的公认知识,这项工作进一步证明,与单金属相比,双金属PtFe位可以促进H 2活化离解,从而加速氢化反应。铂/铈2催化剂。同位素和动力学实验研究溶剂效应的结果表明,可以排除以异丙醇为氢供体的催化转移加氢的反应途径。结果还表明,在反应过程中很容易发生异丙醇与氢和FA的氢交换过程。此外,糠醛氢化可能受到溶剂分子间氢键相互作用的显着影响。
更新日期:2020-08-24
中文翻译:

PtFe双金属催化糠醛选择性氢化为糠醇:异丙醇的溶剂作用和氢活化
负载在CeO 2上的PtFe合金纳米粒子有效地催化了糠醛的高选择性液相加氢。在Pt 3 Fe / CeO 2催化剂上,初始反应速率约为1500 h –1,对糠醇(FA)的选择性接近100%,并且循环稳定性令人满意。基于对铁添加剂对Pt位的几何和电子性质的改性作用的公认知识,这项工作进一步证明,与单金属相比,双金属PtFe位可以促进H 2活化离解,从而加速氢化反应。铂/铈2催化剂。同位素和动力学实验研究溶剂效应的结果表明,可以排除以异丙醇为氢供体的催化转移加氢的反应途径。结果还表明,在反应过程中很容易发生异丙醇与氢和FA的氢交换过程。此外,糠醛氢化可能受到溶剂分子间氢键相互作用的显着影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号