当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidation of Ru/Ce0.5Zr0.5O2–x at Ambient Temperature as a Trigger for Carbon-Free H2 Production by Ammonia Oxidative Decomposition
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-07-31 , DOI: 10.1021/acssuschemeng.0c04126 Takahiro Matsunaga 1 , Suguru Matsumoto 2 , Ryo Tasaki 2 , Yuta Ogura 1 , Takaaki Eboshi 2 , Yuma Takeishi 2 , Kyoko Honda 2 , Katsutoshi Sato 1, 3 , Katsutoshi Nagaoka 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-07-31 , DOI: 10.1021/acssuschemeng.0c04126 Takahiro Matsunaga 1 , Suguru Matsumoto 2 , Ryo Tasaki 2 , Yuta Ogura 1 , Takaaki Eboshi 2 , Yuma Takeishi 2 , Kyoko Honda 2 , Katsutoshi Sato 1, 3 , Katsutoshi Nagaoka 1
Affiliation
|
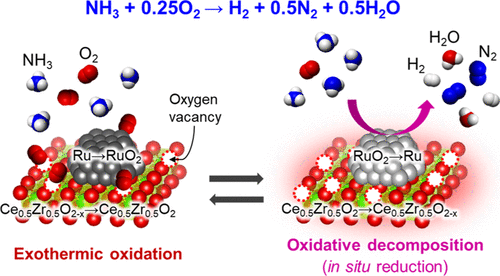
Ammonia has received attention as a hydrogen carrier for energy use, and although ammonia decomposition can produce hydrogen at a high rate, there is currently no simple process for decomposing ammonia that is easily and rapidly initiated at any time without an input of external energy. Here, we report the discovery of a process for initiating and sustaining the production of hydrogen from ammonia without heating the catalyst externally. The oxidative decomposition of ammonia to produce hydrogen at a high rate was repeatedly triggered at ambient temperature (∼25 °C) immediately after NH3 and O2 were supplied to a Ru/Ce0.5Zr0.5O2 catalyst that had been previously reduced at ambient temperature. For this catalyst, active hydrogen atoms formed on Ru0 nanoparticles reduced Ce0.5Zr0.5O2 even at ambient temperature, and the very exothermic oxidation of Ce0.5Zr0.5O2–x as well as Ru0 heated the catalyst bed to the catalytic auto-ignition temperature for ammonia combustion, which was lowered by the contribution of mobile (active) oxygen over the Ce0.5Zr0.5O2. Oxidative ammonia decomposition was thereby triggered.
中文翻译:

室温下Ru / Ce 0.5 Zr 0.5 O 2– x的氧化引发氨氧化分解生成无碳H 2
氨作为能源的氢载体已受到关注,尽管氨分解可以高速率产生氢气,但目前尚无简单的分解氨的方法,该方法可在任何时间,无需外部能量的情况下轻松,迅速地引发。在这里,我们报告了一种在不外部加热催化剂的情况下引发并维持由氨产生氢的过程的发现。向Ru / Ce 0.5 Zr 0.5 O 2供应NH 3和O 2之后,立即在环境温度(约25°C)下反复触发氨的氧化分解,以高速率产生氢气。先前已在环境温度下还原过的催化剂。对于这种催化剂,即使在环境温度下,Ru 0纳米颗粒上形成的活性氢原子也会还原Ce 0.5 Zr 0.5 O 2,并且Ce 0.5 Zr 0.5 O 2– x和Ru 0的非常放热的氧化将催化剂床加热到催化氨燃烧的自燃温度,由于移动(活性)氧对Ce 0.5 Zr 0.5 O 2的贡献而降低。由此触发氧化氨分解。
更新日期:2020-09-08
中文翻译:

室温下Ru / Ce 0.5 Zr 0.5 O 2– x的氧化引发氨氧化分解生成无碳H 2
氨作为能源的氢载体已受到关注,尽管氨分解可以高速率产生氢气,但目前尚无简单的分解氨的方法,该方法可在任何时间,无需外部能量的情况下轻松,迅速地引发。在这里,我们报告了一种在不外部加热催化剂的情况下引发并维持由氨产生氢的过程的发现。向Ru / Ce 0.5 Zr 0.5 O 2供应NH 3和O 2之后,立即在环境温度(约25°C)下反复触发氨的氧化分解,以高速率产生氢气。先前已在环境温度下还原过的催化剂。对于这种催化剂,即使在环境温度下,Ru 0纳米颗粒上形成的活性氢原子也会还原Ce 0.5 Zr 0.5 O 2,并且Ce 0.5 Zr 0.5 O 2– x和Ru 0的非常放热的氧化将催化剂床加热到催化氨燃烧的自燃温度,由于移动(活性)氧对Ce 0.5 Zr 0.5 O 2的贡献而降低。由此触发氧化氨分解。































 京公网安备 11010802027423号
京公网安备 11010802027423号